Proteopedia:Featured EDU/1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<tr><td>[[Image:Phi-psi-clashes-1.png|center|300px]]</td></tr> | <tr><td>[[Image:Phi-psi-clashes-1.png|center|300px]]</td></tr> | ||
<tr><td>'''Tutorial: The Ramachandran principle, phi (φ) and psi (ψ) angles in proteins'''</td></tr> | <tr><td>'''Tutorial: The Ramachandran principle, phi (φ) and psi (ψ) angles in proteins'''</td></tr> | ||
| - | <tr><td>''Eric Martz''</td></tr> | + | <tr><td>''by Eric Martz''</td></tr> |
<tr><td> | <tr><td> | ||
<div class="scrolling"> | <div class="scrolling"> | ||
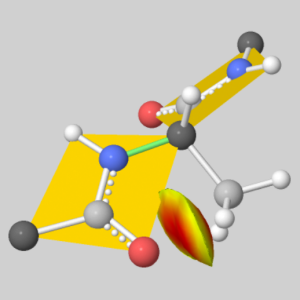
The Ramachandran Principle says that alpha helices, beta strands, and turns are the most likely conformations for a polypeptide chain to adopt, because most other conformations are impossible due to steric collisions between atoms. | The Ramachandran Principle says that alpha helices, beta strands, and turns are the most likely conformations for a polypeptide chain to adopt, because most other conformations are impossible due to steric collisions between atoms. | ||
| - | Check Show Clashes to see where non-bonded atoms are overlapping, and thus in physically impossible positions. | + | Check Show Clashes to see where non-bonded atoms are overlapping, and thus in physically impossible positions. |
</div> | </div> | ||
</td></tr> | </td></tr> | ||
</table> | </table> | ||
Revision as of 11:21, 18 October 2018
| Tutorial: The Ramachandran principle, phi (φ) and psi (ψ) angles in proteins |
| by Eric Martz |
|
The Ramachandran Principle says that alpha helices, beta strands, and turns are the most likely conformations for a polypeptide chain to adopt, because most other conformations are impossible due to steric collisions between atoms. Check Show Clashes to see where non-bonded atoms are overlapping, and thus in physically impossible positions. |