Proteopedia:Featured JRN/1
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<tr><td><div class="scrolling">'''Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in Salmonella enterica.'''<br> | <tr><td><div class="scrolling">'''Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in Salmonella enterica.'''<br> | ||
''Hideyuki Matsunami, Young-Ho Yoon, Vladimir Meshcheryakov, Keiichi Namba, and Fadel A. Samatey''<br>Scientific Reports 6:27399 2016 doi: [http://dx.doi.org/10.1038/srep27399 10.1038/srep27399]<br> | ''Hideyuki Matsunami, Young-Ho Yoon, Vladimir Meshcheryakov, Keiichi Namba, and Fadel A. Samatey''<br>Scientific Reports 6:27399 2016 doi: [http://dx.doi.org/10.1038/srep27399 10.1038/srep27399]<br> | ||
| - | A periplasmic flagellar chaperone protein, FlgA, is required for P-ring assembly in bacterial flagella of taxa such as Salmonella enterica or Escherichia coli. The mechanism of chaperone-mediated P-ring formation is poorly understood. Here we present the open and closed crystal structures of FlgA from Salmonella enterica serovar Typhimurium, grown under different crystallization conditions. An intramolecular disulfide cross-linked form of FlgA caused a dominant negative effect on motility of the wild-type strain.</div></td></tr> | + | A periplasmic flagellar chaperone protein, FlgA, is required for P-ring assembly in bacterial flagella of taxa such as Salmonella enterica or Escherichia coli. The mechanism of chaperone-mediated P-ring formation is poorly understood. Here we present the open and closed crystal structures of FlgA from Salmonella enterica serovar Typhimurium, grown under different crystallization conditions. An intramolecular disulfide cross-linked form of FlgA caused a dominant negative effect on motility of the wild-type strain. |
| + | |||
| + | >>> [[Journal:JBIC:2|Visit this I3DC complement]] >>> | ||
| + | </div> | ||
| + | </td></tr> | ||
</table> | </table> | ||
| + | |||
| + | [[Category:Featured in I3DC]] | ||
Revision as of 15:07, 18 October 2018
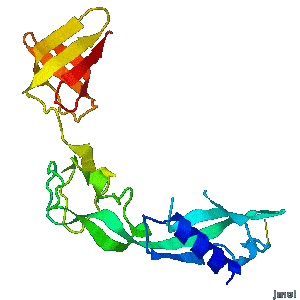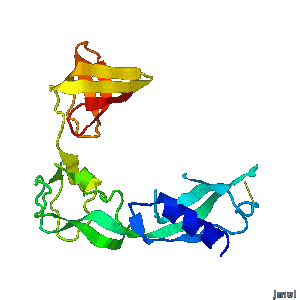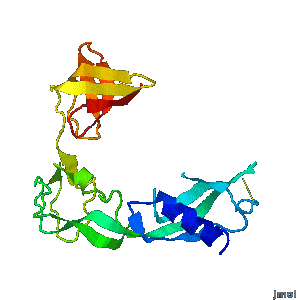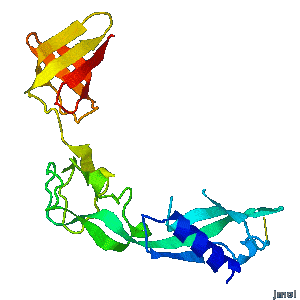 |
Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in Salmonella enterica.
Hideyuki Matsunami, Young-Ho Yoon, Vladimir Meshcheryakov, Keiichi Namba, and Fadel A. Samatey >>> Visit this I3DC complement >>> |
