We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1480
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
[[Image:HAD.PNG]] | [[Image:HAD.PNG]] | ||
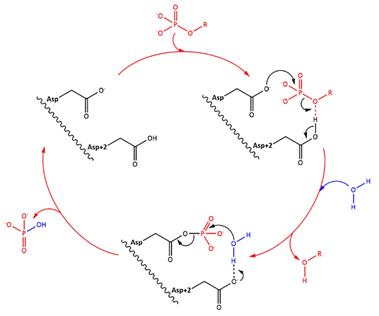
| - | Fig 1. Simplified catalytic mechanism of HAD phosphatases. Asp acts as a nucleophile and Asp+2 as a acid/base. The phosphorylated substrate and the respective leaving groups are shown in red, and the water nucleophile is in blue. (Source: Do metabolic HAD phosphatases moonlight as protein phosphatases?, A. Gohla, 2018). In the human LHPP, the Asp+2 is replaced by a Serine. | + | ''Fig 1. Simplified catalytic mechanism of HAD phosphatases. Asp acts as a nucleophile and Asp+2 as a acid/base. The phosphorylated substrate and the respective leaving groups are shown in red, and the water nucleophile is in blue. (Source: Do metabolic HAD phosphatases moonlight as protein phosphatases?, A. Gohla, 2018). In the human LHPP, the Asp+2 is replaced by a Serine.'' |
Expressed in brain (highest expression level in C1 segment of cervical spinal cord), and at lower levels in liver and kidney. | Expressed in brain (highest expression level in C1 segment of cervical spinal cord), and at lower levels in liver and kidney. | ||
Revision as of 14:49, 7 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Structure of the protein LHPP
| |||||||||||
References
"Molecular cloning of a cDNA for the human phospholysine phosphohistidine inorganic pyrophosphate phosphatase." Yokoi F., Hiraishi H., Izuhara K. J. Biochem. 133:607-614(2003) [PubMed] [Europe PMC] [Abstract]
"The protein histidine phosphatase LHPP is a tumour suppressor." Sravanth K. Hindupur, Marco Colombi, Stephen R. Fuhs, Matthias S. Matter, Yakir Guri, Kevin Adam, Marion Cornu, Salvatore Piscuoglio, Charlotte K. Y. Ng, Charles Betz, Dritan Liko, Luca Quagliata, Suzette Moes, Paul Jenoe, Luigi M. Terracciano, Markus H. Heim, Tony Hunter & Michael N. Hall. (2018) [PubMed] [Main]