Sandbox Reserved 1496
From Proteopedia
| Line 8: | Line 8: | ||
This subpathway is part of the pathway staphyloxanthin biosynthesis, which is itself part of carotenoid biosynthesis. Carotenoid pathways are branches of the general isoprenoid pathway. | This subpathway is part of the pathway staphyloxanthin biosynthesis, which is itself part of carotenoid biosynthesis. Carotenoid pathways are branches of the general isoprenoid pathway. | ||
| - | Staphyloxanthin is a carotenoid, which is responsible for the golden color of S. aureus, and also play the role of virulence factor : it has an antioxidant action that helps the microbe evade death by reactive oxygen species produced by the host immune system. Having a better comprehension of the synthesis of this carotenoid will help to find a cure to S. aureus related diseases. | + | Staphyloxanthin is a carotenoid, which is responsible for the golden color of S. aureus, and also play the role of virulence factor : it has an antioxidant action that helps the microbe evade death by reactive oxygen species produced by the host immune system. Having a better comprehension of the synthesis of this carotenoid will help to find a cure to S. aureus related diseases. {{cn}} |
| - | (The dehydrosqualene (C30 carotene) synthase CrtM is a bacterial carotenoid synthase which is involved in staphyloxanthin synthesis in ''Staphylococcus aureus''.) | + | (The dehydrosqualene (C30 carotene) synthase CrtM is a bacterial carotenoid synthase which is involved in staphyloxanthin synthesis in ''Staphylococcus aureus''.{{cn}}) |
== Function(Reaction) == | == Function(Reaction) == | ||
| - | Catalyzes the head-to-head condensation of two molecules of farnesyl diphosphate (FPP) into the colorless C(30) carotenoid 4,4'-diapophytoene (dehydrosqualene). | + | Catalyzes the head-to-head condensation of two molecules of farnesyl diphosphate (FPP) into the colorless C(30) carotenoid 4,4'-diapophytoene (dehydrosqualene).{{cn}} |
[[Image:CrtMcatalysa.png]] | [[Image:CrtMcatalysa.png]] | ||
| Line 21: | Line 21: | ||
Transferase Activity : Transferring alkyl or aryl groups, other than methyl groups | Transferase Activity : Transferring alkyl or aryl groups, other than methyl groups | ||
| - | Metal Ion Binding : requires | + | Metal Ion Binding : requires Mn<sup>2+</sup> as cofactor (2 ions per subunit). |
== Structure == | == Structure == | ||
| - | This enzyme consists of 2 sequence-identical polypeptide chains of 287 amino acids. | + | This enzyme consists of 2 sequence-identical polypeptide chains of 287 amino acids<ref name="CGSo">[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4866860/]</ref>. |
| - | Ligands : 6 Magnesium ions | + | ; Ligands |
| + | : 6 Magnesium ions | ||
| + | : 2 PO<sub>4</sub><sup>2-</sup> | ||
| + | : 1 L(+)-tartaric acid | ||
| + | : 2 (3r)-3-biphenyl-4-yl-1-azabicyclo[2.2.2]octan-3-ol | ||
Structures of BPH-651, a 3-OH quinuclidine inhibitor, bound to CrtM | Structures of BPH-651, a 3-OH quinuclidine inhibitor, bound to CrtM | ||
| Line 44: | Line 48: | ||
The Staphyloxanthin catalyzed by CrtM is a virulence factor linked with infections caused by methicillin-resistant ''Staphylococcus aureus'' (MRSA) <ref name="ImrS">[https://www.ncbi.nlm.nih.gov/pubmed/17940231]</ref>. Due to its structure similiance as well as shared inhibitor with human squalene synthase (SQS), it is seen as a potential target for virulence factor neutralization therapy, which might reduce the death rate caused by antibiotic resistant bacteria infection. | The Staphyloxanthin catalyzed by CrtM is a virulence factor linked with infections caused by methicillin-resistant ''Staphylococcus aureus'' (MRSA) <ref name="ImrS">[https://www.ncbi.nlm.nih.gov/pubmed/17940231]</ref>. Due to its structure similiance as well as shared inhibitor with human squalene synthase (SQS), it is seen as a potential target for virulence factor neutralization therapy, which might reduce the death rate caused by antibiotic resistant bacteria infection. | ||
| - | Cationic inhibitors are here of interest as | + | Cationic inhibitors are here of interest as anti-infectives because they can substitute themselves to Mg2+ and inactivate the enzyme. |
== References == | == References == | ||
{{reflist}} | {{reflist}} | ||
Revision as of 13:25, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
|
Contents |
Presentation of dehydrosqualene synthase(Function)
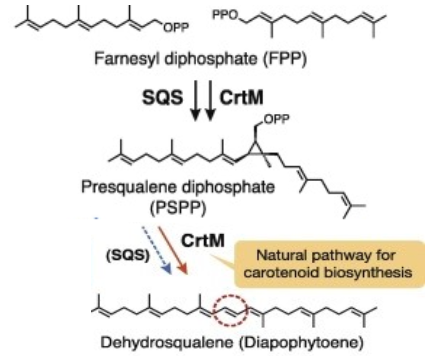
The C30 carotene synthase CrtM enzyme is a bacterial carotenoid synthases which is involved in the first step of the subpathway that synthesizes staphyloxanthin from farnesyl diphosphate.
This subpathway is part of the pathway staphyloxanthin biosynthesis, which is itself part of carotenoid biosynthesis. Carotenoid pathways are branches of the general isoprenoid pathway.
Staphyloxanthin is a carotenoid, which is responsible for the golden color of S. aureus, and also play the role of virulence factor : it has an antioxidant action that helps the microbe evade death by reactive oxygen species produced by the host immune system. Having a better comprehension of the synthesis of this carotenoid will help to find a cure to S. aureus related diseases. Template:Cn
(The dehydrosqualene (C30 carotene) synthase CrtM is a bacterial carotenoid synthase which is involved in staphyloxanthin synthesis in Staphylococcus aureus.Template:Cn)
Function(Reaction)
Catalyzes the head-to-head condensation of two molecules of farnesyl diphosphate (FPP) into the colorless C(30) carotenoid 4,4'-diapophytoene (dehydrosqualene).Template:Cn
Transferase Activity : Transferring alkyl or aryl groups, other than methyl groups
Metal Ion Binding : requires Mn2+ as cofactor (2 ions per subunit).
Structure
This enzyme consists of 2 sequence-identical polypeptide chains of 287 amino acids[1].
- Ligands
- 6 Magnesium ions
- 2 PO42-
- 1 L(+)-tartaric acid
- 2 (3r)-3-biphenyl-4-yl-1-azabicyclo[2.2.2]octan-3-ol
Structures of BPH-651, a 3-OH quinuclidine inhibitor, bound to CrtM
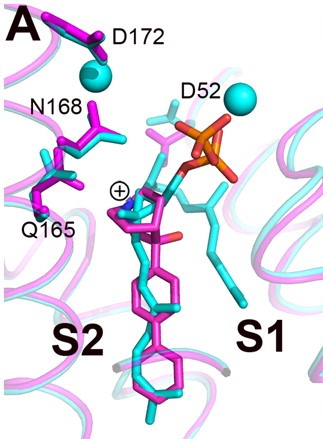
The space group of FsPP·CrtM is P3121, with two molecules per asymmetric unit[2]. Three phosphonosulfonates, which mimic the FPP substract have been identified to have binding/inhibiting activity on CrtM, each with different binding pattern.
Biological process
CrtM catalyses the first step of the synthesis of staphyloxanthin (with a 2-step mechanism)[3]. The catalysed reaction is : 2 (2E,6E)-farnesyl diphosphate <=> 15-cis-4,4'-diapophytoene + 2 diphosphate
Relevance to diseases
The Staphyloxanthin catalyzed by CrtM is a virulence factor linked with infections caused by methicillin-resistant Staphylococcus aureus (MRSA) [4]. Due to its structure similiance as well as shared inhibitor with human squalene synthase (SQS), it is seen as a potential target for virulence factor neutralization therapy, which might reduce the death rate caused by antibiotic resistant bacteria infection. Cationic inhibitors are here of interest as anti-infectives because they can substitute themselves to Mg2+ and inactivate the enzyme.