We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1507
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
==3D2F, Crystal structure of a complex of Sse1p and Hsp70== | ==3D2F, Crystal structure of a complex of Sse1p and Hsp70== | ||
| - | <StructureSection load='3d2f' size=' | + | <StructureSection load='3d2f' size='500' side='right' caption='Caption for this structure' scene=''> |
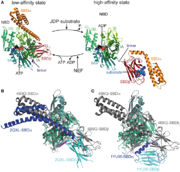
'''3D2F''' is a complex formed with two proteins '''Sse1p''' and '''Hsp70'''. Both have a role in helping protein folding. The interaction of Hsp70 with Sse1p catalyse the liberation of Hsp70s’ ADP and triggers the good folding of the substrat. | '''3D2F''' is a complex formed with two proteins '''Sse1p''' and '''Hsp70'''. Both have a role in helping protein folding. The interaction of Hsp70 with Sse1p catalyse the liberation of Hsp70s’ ADP and triggers the good folding of the substrat. | ||
| Line 45: | Line 45: | ||
The Hsp70’s PDB which is tightly linked with Sse1p ATP-bound NBD is considered to be the part of the protein helping in the remodelling of misfolded proteins. During this step Hsp70 ADP is liberated. It is the formation of a new bond between Hsp70 and an other ATP that may trigger the dissociation of the complex and the partial or complete folding of the protein. If the protein has not been completely folded, it can bind again to Hsp70 for a new folding cycle. | The Hsp70’s PDB which is tightly linked with Sse1p ATP-bound NBD is considered to be the part of the protein helping in the remodelling of misfolded proteins. During this step Hsp70 ADP is liberated. It is the formation of a new bond between Hsp70 and an other ATP that may trigger the dissociation of the complex and the partial or complete folding of the protein. If the protein has not been completely folded, it can bind again to Hsp70 for a new folding cycle. | ||
| - | [[Image:Image-Animated 3d2f.gif]] | ||
When interacting with Hsp70, Sse1p’s NBD is bound with ATP. It’s NBD and 3HBD are tightly interacting with the NBD of the Hsp70. | When interacting with Hsp70, Sse1p’s NBD is bound with ATP. It’s NBD and 3HBD are tightly interacting with the NBD of the Hsp70. | ||
Revision as of 19:37, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
3D2F, Crystal structure of a complex of Sse1p and Hsp70
| |||||||||||
References
Frontiers in Molecular Biosciences, Insights into the molecular mechanism of allostery in Hsp70s https://www.frontiersin.org/articles/10.3389/fmolb.2015.00058/full
Structural Basis for the Cooperation of Hsp70 and Hsp110 Chaperones in Protein Folding
Sigrun Polier, Zdravko Dragovic, F. Ulrich Harti, Andreas Bracher
Cell 133, 1068-1079, June 13, 2008
[PubMed Abstract] Ballinger, C. A., Connell, P., Wu, Y., Hu, Z., Thompson, L. J., Yin, L. Y., et al. (1999). Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19, 4535–4545.