We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1507
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
The Hsp110 family is composed of two proteins Sse1p and Sse2p. Sse1p is constitutively expressed while the production of Sse2p is stress dependant. | The Hsp110 family is composed of two proteins Sse1p and Sse2p. Sse1p is constitutively expressed while the production of Sse2p is stress dependant. | ||
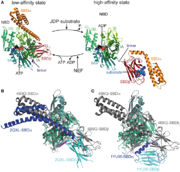
| - | Hsp110 proteins are homologous to Hsp70. The general domain organisation of Sse1p ressemble to one of the canonical Hsp70s. They are composed of different domains: a N-terminal actin type Nucleotide-Binding Domain, a ß sandwich domain and a C-terminal 3 helix bundle domain (3HBD). | + | Hsp110 proteins <ref> DOI: 10.1515/hsz-2018-0209 </ref> are homologous to Hsp70. The general domain organisation of Sse1p ressemble to one of the canonical Hsp70s. They are composed of different domains: a N-terminal actin type Nucleotide-Binding Domain, a ß sandwich domain and a C-terminal 3 helix bundle domain (3HBD). |
All the interaction with the NBD of Hsp70 is located along the NBD and 3HBD of the Sse1p. The interaction with Hsp70 only causes minor changes in its conformation. | All the interaction with the NBD of Hsp70 is located along the NBD and 3HBD of the Sse1p. The interaction with Hsp70 only causes minor changes in its conformation. | ||
Revision as of 13:53, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
3D2F, Crystal structure of a complex of Sse1p and Hsp70
| |||||||||||
References
- ↑ Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002 Mar 8;295(5561):1852-8. doi: 10.1126/science.1068408. PMID:11884745 doi:http://dx.doi.org/10.1126/science.1068408
- ↑ Polier S, Dragovic Z, Hartl FU, Bracher A. Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell. 2008 Jun 13;133(6):1068-79. PMID:18555782 doi:10.1016/j.cell.2008.05.022
- ↑ Mayer MP, Kityk R. Insights into the molecular mechanism of allostery in Hsp70s. Front Mol Biosci. 2015 Oct 20;2:58. doi: 10.3389/fmolb.2015.00058. eCollection, 2015. PMID:26539440 doi:http://dx.doi.org/10.3389/fmolb.2015.00058
- ↑ Yakubu UM, Morano KA. Roles of the nucleotide exchange factor and chaperone Hsp110 in cellular proteostasis and diseases of protein misfolding. Biol Chem. 2018 Sep 25;399(10):1215-1221. doi: 10.1515/hsz-2018-0209. PMID:29908125 doi:http://dx.doi.org/10.1515/hsz-2018-0209
- ↑ Polier S, Dragovic Z, Hartl FU, Bracher A. Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell. 2008 Jun 13;133(6):1068-79. PMID:18555782 doi:10.1016/j.cell.2008.05.022