User:Estelle Blochouse/ Sandbox 1497
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
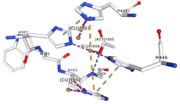
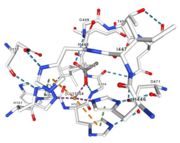
Multicopper oxidases are able to oxidise their substrates thanks to their particular structure. They own four copper ions (Cu1001, Cu1002, Cu1003 and C1004) dispatched between two important areas : the mononuclear copper center (Cu1001) and the trinuclear copper center (Cu1002, Cu1003 and Cu1004). | Multicopper oxidases are able to oxidise their substrates thanks to their particular structure. They own four copper ions (Cu1001, Cu1002, Cu1003 and C1004) dispatched between two important areas : the mononuclear copper center (Cu1001) and the trinuclear copper center (Cu1002, Cu1003 and Cu1004). | ||
| - | + | ||
== Mechanism == | == Mechanism == | ||
Multicopper oxidase catalyzes the oxidation of different substrates by reducing O2 into H2O without releasing activated oxygen species (H2O2). | Multicopper oxidase catalyzes the oxidation of different substrates by reducing O2 into H2O without releasing activated oxygen species (H2O2). | ||
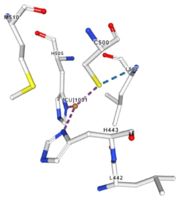
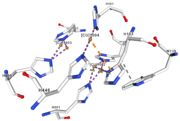
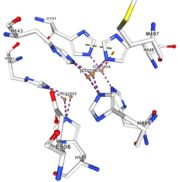
| - | Three differents copper centers exist that can be differentiated spectroscopically: Type 1 or blue (<scene name='pdbligand=CU:COPPER+(II)+ION'>mononoclear copper center</scene>), type 2 or normal and type 3 or coupled binuclear.<ref>Messerschmidt A, Huber R, The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships, Eur. J. Biochem. 187, January 1990</ref><ref>Ouzounis C, Sander C, A structure-derived sequence pattern for the detection of type I copper binding domains in distantly related proteins, FEBS Lett. volume 279, February 1991</ref>. Multicopper oxidase contains these 3 types of copper ions. They are all involved in the transfer of electrons from the substrate to the dioxygen. The first type of copper, type 1, (Cu1001) mediates the electron transfer from the substrate to the other coppers. The <scene name='pdbligand=CU:COPPER+(II)+ION'>mononoclear copper center</scene> formed by the three other coppers is the key element for the oxygen reduction. It contains a type 2 copper (Cu1004) and two type 3 copper ions, binuclear ions, (Cu1002 and Cu1003). The final electron acceptor, O2, is bound to this last type of copper and is reduced into two molecules of water thanks to the | + | Three differents copper centers exist that can be differentiated spectroscopically: Type 1 or blue (<scene name='pdbligand=CU:COPPER+(II)+ION'>mononoclear copper center</scene>), type 2 or normal and type 3 or coupled binuclear.<ref>Messerschmidt A, Huber R, The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships, Eur. J. Biochem. 187, January 1990</ref><ref>Ouzounis C, Sander C, A structure-derived sequence pattern for the detection of type I copper binding domains in distantly related proteins, FEBS Lett. volume 279, February 1991</ref>. |
| + | Multicopper oxidase contains these 3 types of copper ions. They are all involved in the transfer of electrons from the substrate to the dioxygen. The first type of copper, type 1, (Cu1001) mediates the electron transfer from the substrate to the other coppers. The <scene name='pdbligand=CU:COPPER+(II)+ION'>mononoclear copper center</scene> formed by the three other coppers is the key element for the oxygen reduction. It contains a type 2 copper (Cu1004) and two type 3 copper ions, binuclear ions, (Cu1002 and Cu1003). The final electron acceptor, O2, is bound to this last type of copper and is reduced into two molecules of water thanks to the transfer of four electrons allowed by the oxidation of the four copper ions.<ref>Hirofumi Komori, Ryosuke | ||
Sugiyama, Kunishige Kataoka, | Sugiyama, Kunishige Kataoka, | ||
Kentaro Miyazaki, Yoshiki | Kentaro Miyazaki, Yoshiki | ||
Revision as of 20:00, 11 January 2019
Multicopper Oxidase CueO (4e9s)
| |||||||||||
References
- ↑ EMBL-EBI, Family: Cu-oxidase (PF00394), Summary: Multicopper oxidase, http://pfam.xfam.org/family/Cu-oxidase
- ↑ UniProtKB
- ↑ Messerschmidt A, Huber R, The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships, Eur. J. Biochem. 187, January 1990
- ↑ Ouzounis C, Sander C, A structure-derived sequence pattern for the detection of type I copper binding domains in distantly related proteins, FEBS Lett. volume 279, February 1991
- ↑ Hirofumi Komori, Ryosuke Sugiyama, Kunishige Kataoka, Kentaro Miyazaki, Yoshiki Higuchib, and Takeshi Sakurai, New insights into the catalytic active-site structure of multicopper oxidases, Biological Crystallography, 6 December 2013 doi:10.1107/S1399004713033051
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ Kataoka K, Komori H, Ueki Y, Konno Y, Kamitaka Y, Kurose S, Tsujimura S, Higuchi Y, Kano K, Seo D, Sakurai T. Structure and function of the engineered multicopper oxidase CueO from Escherichia coli--deletion of the methionine-rich helical region covering the substrate-binding site. J Mol Biol. 2007 Oct 12;373(1):141-52. Epub 2007 Aug 2. PMID:17804014 doi:10.1016/j.jmb.2007.07.041