Introduction
Acetylcholinesterase (AChE) is essential for hydrolysis of the neurotransmitter acetylcholine (ACh), and, therefore, for termination of impulse transmission at cholinergic synapses (Figure 2). Irreversible inhibition of AChE can result in accumulation of ACh at cholinergic synapses and, ultimately, to death. Conversely, decreased levels of ACh may result in the memory deficits associated with Alzheimer's disease[1]. AChE has a deep (20Å) and narrow (5Å) gorge lined with 14 aromatic residues, with its active site located near the bottom of the gorge[2]. Initially, ACh binds to the peripheral anionic site (PAS) of AChE, and is funneled down the gorge to the active site by interactions between its quaternary ammonium group and the aromatic rings of 14 aromatic amino acid residues lining the gorge. At the active site, ACh is oriented for hydrolysis by interactions between the catalytic anionic site and its quaternary ammonium group. Fasciculin-II (FAS-II), a potent polypeptide toxin present in the venom of the East African green mamba (Dendroaspis angusticeps), inhibits AChE by binding to the top of the active-site gorge, interacting tightly with residues that form the PAS; it thus prevents ACh from entering the active-site gorge[3]. The Hostos-Lincoln Academy Students Modeling A Research Topic (S.M.A.R.T) team and the Center for BioMolecular Modeling have designed and fabricated two physical models using a combination of computational molecular modeling and three-dimensional (3D) printing technology: Torpedo californica (Tc) AChE complexed with a modeled ACh molecule ligand, and a complex of FAS-II with TcAChE.
Background Information
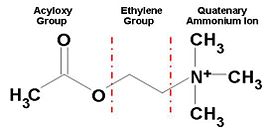
Figure 1. Chemical Structure of Acetylcholine
When a nerve impulse reaches the presynaptic nerve terminal of a cholinergic synpase, it stimulates the release of the neurotransmitter, ACh (Figure 1), into the synaptic cleft. ACh diffuses across the cleft to the postsynaptic nerve terminal, where it binds reversibly to acetylcholine receptors embedded in the membrane of the postsynaptic nerve terminal. The binding of ACh to the receptors triggers a nerve impulse in the postsynaptic neuron. Finally AChE, anchored to the membrane of the postsynaptic nerve terminal (Figure 2), hydrolyzes ACh to acetate and choline, resulting in the termination of neurotransmission.
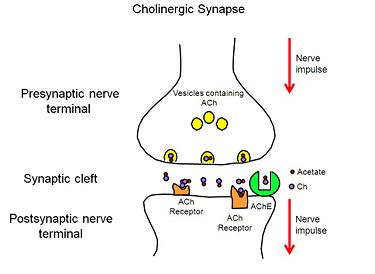
Figure 2. Cholinergic Synapse
Inhibition of AChE may result in various outcomes, depending on the physiological context. Toxins such as FAS-II, from the green mamba, a poisonous snake found in East Africa, inhibit AChE and ultimately lead to death. However, controlled inhibition of AChE, in patients with Alzheimer’s disease, by drugs designed for this purpose, alleviates their symptoms, including memory loss and disorientation.
Models of AChE
(2ace) (1fss)
Designing Physical Models to Tell the Story of Acetylcholinesterase
Reflected in our design are two key concepts of AChE biology: the mechanism by which AChE hydrolyses ACh (the substrate traffic story), and how the Green Mamba Snake toxin, FAS-II, inhibits the hydrolysis of ACh (the inhibition story)[4]. Two physical models were designed and fabricated using a combination of computational molecular modeling and 3D printing technology: TcAChE in complex with a modeled ACh ligand, and TcAChE in complex with FAS-II. Both models were designed using the respective protein data bank (PDB) files: 2ace for the TcAChE/ACh complex and 1fss for theTcAChE/FAS-II complex, and RasMol computer modeling program.
Features of the Substrate Traffic Story: a Model of AChE/ACh
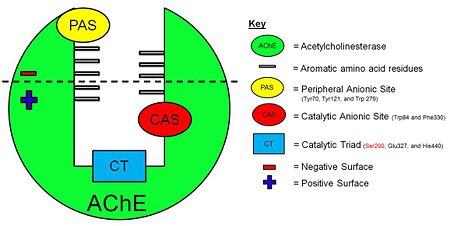
Figure 3. Schematic illustration of AChE.
Model of
The Tc protein contains 537 amino acids and forms an α/β hydrolase fold. The neurotransmitter consists of an acytoxy group, an ethylene group and a positively charged quaternary ammonium ion.
The that line the active site gorge are Tyr70, Trp84, Trp120, Tyr121, Tyr130, Trp233, Trp279, Phe288, Phe290, Phe330, Phe331, Tyr334, Trp432 and Tyr442. These aromatic residues interact with the positively charged quaternary ammonium ion of ACh by virtue of cation-π interactions to filter it down the active-site gorge to the catalytic triad (Figure 3).
The PAS includes residues . Initially, the positively charged quaternary ammonium ion of ACh is attracted to and binds to the , highlighted in yellow.
The Catalytic Anionic Site (CAS) includes residues . The
, highlighted in red, holds ACh in the optimal position for hydrolysis by interacting with the quaternary ammonium ion of ACh.
The AChE active site includes three residues that form a catalytic triad: . The , highlighted in blue, is responsible for the hydrolysis of ACh into acetate and choline.
Features of the Inhibition Story: a Model of AChE/FAS-II
Model of
The Green Mamba snake toxin, , is a 61-residue protein that folds into 4β sheets, with 3 of the 4β sheets forming loops, or fingers.
FAS-II binds to and inhibits AChE using two major mechanisms:
1. Long-range electrostatic complementarity: the positive lower region of FAS-II is attracted to the highly negative top region of AChE (Figure 4).
2. Amino acid specificity: residues are located on two of the three fingers of FAS-II. When FAS-II to AChE, Arg27 and Met33 interact with Trp279 part of the PAS, while Thr8 and Val34 interact with Tyr70, also part of the PAS.
3. Shape: Once bound to the PAS, two loops of FAS-II fit in to the AChE active-site gorge like a hand fits into a glove. Once this occurs, the entrance of the gorge is such that acetylcholine may not enter, and therefore it will not be hydrolysed. This results in the increased levels of AChE in the cholinergic synapse, and ultimately death.
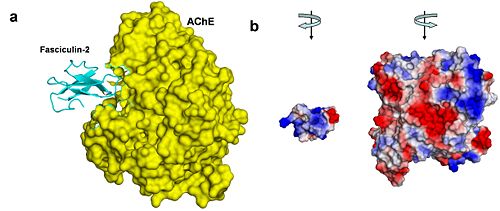
Figure 4. AChE-fasciculin-2 complex. (a) A side view of the complex, illustrating the geometric complementarity of the two interacting proteins. AChE is presented as a yellow surface and fasciculin-2 as a blues ribbon. (b) A front view of both interacting proteins, presented separately as surfaces colored by electrostatic potential (blue is positive, white is neutral, and red is negative). To create this view, both proteins were rotated 90º compared to their position in a, AChE to the right and fasciculin to the left. The electrostatic compatibility between the two proteins is clear; The positively charged part of fasciculin matches the entrance to AChE's binding site, which is negatively charged
[5].
References
- ↑ Greenblatt HM, Dvir H, Silman I, Sussman JL. Acetylcholinesterase: a multifaceted target for structure-based drug design of anticholinesterase agents for the treatment of Alzheimer's disease. J Mol Neurosci. 2003;20(3):369-83. PMID:14501022 doi:10.1385/JMN:20:3:369
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Harel M, Kleywegt GJ, Ravelli RB, Silman I, Sussman JL. Crystal structure of an acetylcholinesterase-fasciculin complex: interaction of a three-fingered toxin from snake venom with its target. Structure. 1995 Dec 15;3(12):1355-66. PMID:8747462
- ↑ Silman I, Sussman JL. Acetylcholinesterase: how is structure related to function? Chem Biol Interact. 2008 Sep 25;175(1-3):3-10. Epub 2008 Jun 6. PMID:18586019 doi:10.1016/j.cbi.2008.05.035
- ↑ Kessel A and Ben-Tal N (Dec. 2010) Introduction to Proteins: Structure, Function, and Motion. Chapman & Hall/CRC Mathematical & Computational Biology. ISBN: 9781439810712






