User:Asif Hossain/Sandbox 1
From Proteopedia
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
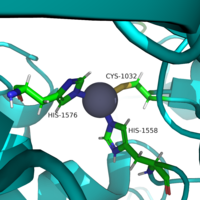
Histone deacetylase 8 (HDAC 8) is an enzyme that plays a role in controlling gene expression. Specifically, it catalyzes the removal of an acetyl group off of the ε-amino-lysine sidechain of N-terminal core of Histone proteins. By removing the acetate ion, the reclaimed positive charge on the lysine sidechain is able to interact with the negative charge on the DNA. As a result, DNA will bind more tightly to the histone protein reducing transcription and expression. | Histone deacetylase 8 (HDAC 8) is an enzyme that plays a role in controlling gene expression. Specifically, it catalyzes the removal of an acetyl group off of the ε-amino-lysine sidechain of N-terminal core of Histone proteins. By removing the acetate ion, the reclaimed positive charge on the lysine sidechain is able to interact with the negative charge on the DNA. As a result, DNA will bind more tightly to the histone protein reducing transcription and expression. | ||
| - | + | ||
| - | + | ||
| - | + | ||
<StructureSection load='2v5w' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='2v5w' size='340' side='right' caption='Caption for this structure' scene=''> | ||
Revision as of 19:27, 7 April 2019
Histone Deacetylase 8 (HDAC 8)
Introduction
Histone deacetylase 8 (HDAC 8) is an enzyme that plays a role in controlling gene expression. Specifically, it catalyzes the removal of an acetyl group off of the ε-amino-lysine sidechain of N-terminal core of Histone proteins. By removing the acetate ion, the reclaimed positive charge on the lysine sidechain is able to interact with the negative charge on the DNA. As a result, DNA will bind more tightly to the histone protein reducing transcription and expression.
| |||||||||||
References
1. Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634.