We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Nicholas Bantz/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
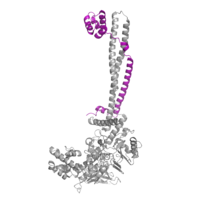
[[Image:COREST.png|200 px|right|thumb|Figure 3: CoRest complex (purple) bound to LSD1 at the Tower domain.]] | [[Image:COREST.png|200 px|right|thumb|Figure 3: CoRest complex (purple) bound to LSD1 at the Tower domain.]] | ||
| - | The <scene name='81/811088/Towerdomain/2'>tower domain</scene> is 100 residue protrusion off of the main protein body of LSD-1 (Figure 2), which form 2 [https://en.wikipedia.org/wiki/Alpha_helix-helices 𝛂-helices]. The longer helix, T𝛂A, is an LSD-1 specific element that has not been found in any other oxidase proteins <ref name="Stavropolous">doi: 10.1038/nsmb1113</ref>. The shorter helix, T𝛂B, is very near to the active site of the oxidase domain. In fact, T𝛂B connects directly to helix 𝛂D of the oxidase domain through a highly conserved connector loop. The exact function of the tower domain is not known, but it is proposed to regulate the size of the active site chamber through this <scene name='81/811090/Tb-dinteraction/1'>TαB-αD interaction</scene>. The T𝛂B-𝛂D interaction is responsible for the proper positioning of <scene name='81/811090/Phe538-tyr761interaction/1'>Phe538</scene>, a side chain of 𝛂D that is located in the catalytic chamber. In addition, the T𝛂B-𝛂D interaction positions 𝛂D in the correct manner to provide [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonding] to <scene name='81/811090/Phe538-tyr761interaction/1'>Tyr761</scene>. Tyr761 is positioned in the catalytic chamber very close to the FAD cofactor, and aids in the binding of the lysine substrate <ref name="Stavropolous"/>. Therefore, the base of the tower domain forms a direct connection to the oxidase domain and plays a crucial role in the shape and catalytic activity of the active site. In fact, removing the tower domain via a mutation resulted in a drastic decrease in catalytic efficiency <ref name="Stavropolous"/>. The tower domain has also been found to interact with other proteins and complexes, such as CoREST (Figure 3), as a molecular lever to allosterically regulate the catalytic activity of the active site <ref name="Yang">doi: 10.1016/j.molcel.2006.07.012</ref>. Overall, the exact function of the tower domain has not yet been determined, but it is known to be vital to the catalytic activity of LSD-1. | + | The <scene name='81/811088/Towerdomain/2'>tower domain</scene> is a 100 residue protrusion off of the main protein body of LSD-1 (Figure 2), which form 2 [https://en.wikipedia.org/wiki/Alpha_helix-helices 𝛂-helices]. The longer helix, T𝛂A, is an LSD-1 specific element that has not been found in any other oxidase proteins <ref name="Stavropolous">doi: 10.1038/nsmb1113</ref>. The shorter helix, T𝛂B, is very near to the active site of the oxidase domain. In fact, T𝛂B connects directly to helix 𝛂D of the oxidase domain through a highly conserved connector loop. The exact function of the tower domain is not known, but it is proposed to regulate the size of the active site chamber through this <scene name='81/811090/Tb-dinteraction/1'>TαB-αD interaction</scene>. The T𝛂B-𝛂D interaction is responsible for the proper positioning of <scene name='81/811090/Phe538-tyr761interaction/1'>Phe538</scene>, a side chain of 𝛂D that is located in the catalytic chamber. In addition, the T𝛂B-𝛂D interaction positions 𝛂D in the correct manner to provide [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonding] to <scene name='81/811090/Phe538-tyr761interaction/1'>Tyr761</scene>. Tyr761 is positioned in the catalytic chamber very close to the FAD cofactor, and aids in the binding of the lysine substrate <ref name="Stavropolous"/>. Therefore, the base of the tower domain forms a direct connection to the oxidase domain and plays a crucial role in the shape and catalytic activity of the active site. In fact, removing the tower domain via a mutation resulted in a drastic decrease in catalytic efficiency <ref name="Stavropolous"/>. The tower domain has also been found to interact with other proteins and complexes, such as CoREST (Figure 3), as a molecular lever to allosterically regulate the catalytic activity of the active site <ref name="Yang">doi: 10.1016/j.molcel.2006.07.012</ref>. Overall, the exact function of the tower domain has not yet been determined, but it is known to be vital to the catalytic activity of LSD-1. |
Revision as of 20:11, 8 April 2019
Human lysine-specific histone demethylase (LSD-l)
| |||||||||||
Student Contributors
- Nicholas Bantz
- Cody Carley
- Michael Thomas