We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Sean Callahan/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
==Regulation== | ==Regulation== | ||
=== Allosteric Stie === | === Allosteric Stie === | ||
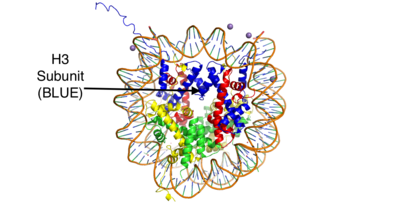
| - | LSD-1 is a protein that can be regulated by outside factors. The tower domain and oxidase domain are connected by a <scene name='81/811711/Regulator_loop/6'>tower oxidase connector</scene>. The oxidase domain is what holds the catalytic chamber for LSD-1. This makes the oxidase domain a very sensitive domain. Any change to the catalytic chamber could drastically reduce the ability for the methylated amine to fit in the binding site correctly. Because the tower domain is attached to the oxidase domain, the tower domain and the connector region become allosteric sites. Any interaction with these two sites is hypothesized to drastically change the enzyme activity. This has further pushed the assumption that the CoRest complex interacts with the tower region | + | LSD-1 is a protein that can be regulated by outside factors. The tower domain and oxidase domain are connected by a <scene name='81/811711/Regulator_loop/6'>tower oxidase connector</scene>. The oxidase domain is what holds the catalytic chamber for LSD-1. This makes the oxidase domain a very sensitive domain. Any change to the catalytic chamber could drastically reduce the ability for the methylated amine to fit in the binding site correctly. Because the tower domain is attached to the oxidase domain, the tower domain and the connector region become allosteric sites. Any interaction with these two sites is hypothesized to drastically change the enzyme activity. This has further pushed the assumption that the CoRest complex interacts with the tower region<ref name="Stavropoulos">PMID: 16799558</ref>. |
===Androgen Receptor=== | ===Androgen Receptor=== | ||
| - | LSD-1 will demethylate mono or di-methylated lysines. However it will not demethylate just any lysine. It will only demethylate lysine H3-K4. This factor can be regulated by the androgen receptor. The [http://proteopedia.org/wiki/index.php/Androgen_receptor#Function androgen receptor] is a protein that is involved in DNA transcription. When it interacts with LSD-1 it will no longer demethylate H3-K4, but will now demethylate H3-K9. This attribute allows LSD-1 to work on a wider range of residues | + | LSD-1 will demethylate mono or di-methylated lysines. However it will not demethylate just any lysine. It will only demethylate lysine H3-K4. This factor can be regulated by the androgen receptor. The [http://proteopedia.org/wiki/index.php/Androgen_receptor#Function androgen receptor] is a protein that is involved in DNA transcription. When it interacts with LSD-1 it will no longer demethylate H3-K4, but will now demethylate H3-K9. This attribute allows LSD-1 to work on a wider range of residues<ref name="Stavropoulos">PMID: 16799558</ref>. |
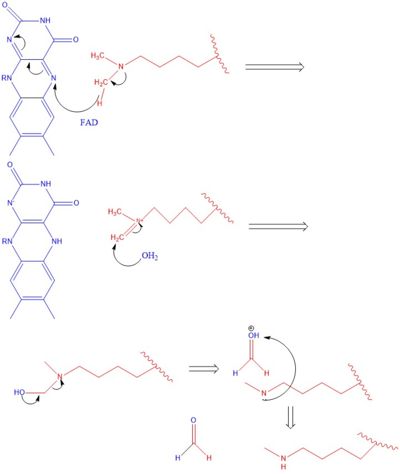
==Mechanism== | ==Mechanism== | ||
| Line 38: | Line 38: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | <ref>PMID:16799558</ref> | ||
<ref name=”Qian">PMID: 16299514 </ref> | <ref name=”Qian">PMID: 16299514 </ref> | ||
<ref name=”Forneris">PMID: 16223729 </ref> | <ref name=”Forneris">PMID: 16223729 </ref> | ||
Revision as of 00:47, 12 April 2019
Lysine Specific Demethylase 1 (Homo sapiens)
| |||||||||||
References
- ↑ 1.0 1.1 Forneris F, Binda C, Vanoni MA, Mattevi A, Battaglioli E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005 Apr 11;579(10):2203-7. doi: 10.1016/j.febslet.2005.03.015. PMID:15811342 doi:http://dx.doi.org/10.1016/j.febslet.2005.03.015
- ↑ Qian C, Zhang Q, Li S, Zeng L, Walsh MJ, Zhou MM. Structure and chromosomal DNA binding of the SWIRM domain. Nat Struct Mol Biol. 2005 Dec;12(12):1078-85. Epub 2005 Nov 20. PMID:16299514 doi:10.1038/nsmb1022
- ↑ 3.0 3.1 Da G, Lenkart J, Zhao K, Shiekhattar R, Cairns BR, Marmorstein R. Structure and function of the SWIRM domain, a conserved protein module found in chromatin regulatory complexes. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2057-62. Epub 2006 Feb 3. PMID:16461455
- ↑ 4.0 4.1 4.2 4.3 4.4 Stavropoulos P, Blobel G, Hoelz A. Crystal structure and mechanism of human lysine-specific demethylase-1. Nat Struct Mol Biol. 2006 Jul;13(7):626-32. Epub 2006 Jun 25. PMID:16799558 doi:10.1038/nsmb1113
- ↑ 5.0 5.1 Abdel-Magid AF. Lysine-Specific Demethylase 1 (LSD1) Inhibitors as Potential Treatment for Different Types of Cancers. ACS Med Chem Lett. 2017 Oct 27;8(11):1134-1135. doi:, 10.1021/acsmedchemlett.7b00426. eCollection 2017 Nov 9. PMID:29152043 doi:http://dx.doi.org/10.1021/acsmedchemlett.7b00426
- ↑ Qian C, Zhang Q, Li S, Zeng L, Walsh MJ, Zhou MM. Structure and chromosomal DNA binding of the SWIRM domain. Nat Struct Mol Biol. 2005 Dec;12(12):1078-85. Epub 2005 Nov 20. PMID:16299514 doi:10.1038/nsmb1022
- ↑ Forneris F, Binda C, Vanoni MA, Battaglioli E, Mattevi A. Human histone demethylase LSD1 reads the histone code. J Biol Chem. 2005 Dec 16;280(50):41360-5. doi: 10.1074/jbc.M509549200. Epub 2005 , Oct 13. PMID:16223729 doi:http://dx.doi.org/10.1074/jbc.M509549200