We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Uncoupling Protein 2
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
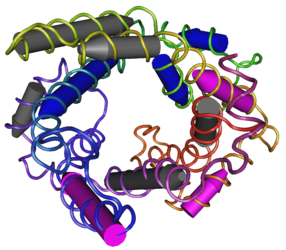
Figure 1: Uncoupling Protein 2 | Figure 1: Uncoupling Protein 2 | ||
| + | |||
| + | == Introduction == | ||
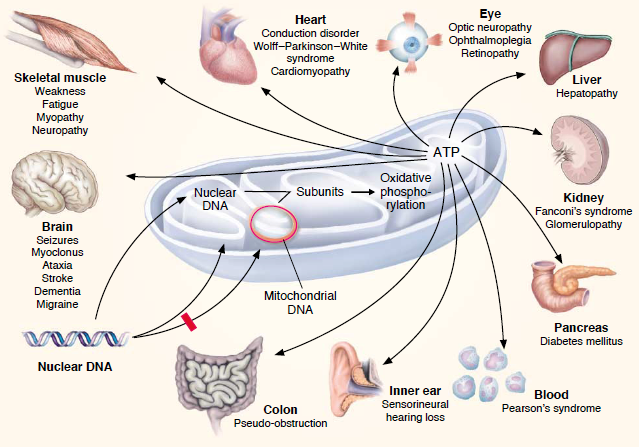
| + | Mutations involving the mitochondria can lead to varies diseases. One particular disease that involves a 3243G mutation in the mitochondrial DNA-encoded tRNA gene is known as Diabetes Mellitus (DM). The predominant issues seen in patients with this disease is glucose intolerances due to gradual development of pancreatic beta-cell malfunction which can be seen with increased age. Uncoupling proteins are key factors that generates these issues. Low levels of uncoupling proteins (UCPs) can lead to high mitochondrial membrane potentials. These increased potentials favor generation of reactive oxygen species (ROS). Increased ROS levels assist the progress of apoptosis and the differentiation state of pancreatic beta-cells which ultimately lead to inadequate secretion of insulin and an inability to maintain glucose levels. Five UCPs have been discovered in mammals, but UCP1, UCP2 and UCP3 have the biggest roles in DM. UPC1’s underlying determinant of DM is from an imbalance of energy intake and expenditure. Research has also found that polymorphisms and UCP1’s mRNA expressions are highly associated with DM. UCP2, the main focus of this research, is known as a negative regulator of insulin secretion and plays the biggest role in DM generation. The ROS initiate UCP2 which is preceded by decreased beta-cell ATP synthesis and lack of regulation of glucose-stimulating insulin secretion. Polymorphism of UCP2 gene is also correlated with DM. UCP3 is also shown to influence insulin secretion through these beta-cells, but less research has been conducted on these proteins. | ||
== Disease == | == Disease == | ||
Revision as of 02:26, 15 April 2019
Uncoupling Protein 2 (UCP2) in Diabetes
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Maassen, J. A.; Leen; Hart; Essen, E. van; Heine, R. J.; Nijpels, G.; Tafrechi, R. S. J.; Raap, A. K.; Janssen, G. M. C.; Lemkes, H. H. P. J. Mitochondrial Diabetes. http://diabetes.diabetesjournals.org/content/53/suppl_1/S103 (accessed Apr 1, 2019).