This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Asif Hossain/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
The crystal structure of human HDAC8 was determined using x-ray crystallography at a 2.0Å resolution. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The structure includes two structural K ion and one catalytic Zn ion. HDAC8 is bound to a [https://en.wikipedia.org/wiki/P53 p-53] derived diacetylated peptide substrate as opposed to the natural histone substrate. This peptide includes a fluorescent coumarin ring likely used in past kinetic assays. | The crystal structure of human HDAC8 was determined using x-ray crystallography at a 2.0Å resolution. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The structure includes two structural K ion and one catalytic Zn ion. HDAC8 is bound to a [https://en.wikipedia.org/wiki/P53 p-53] derived diacetylated peptide substrate as opposed to the natural histone substrate. This peptide includes a fluorescent coumarin ring likely used in past kinetic assays. | ||
| - | The HDAC8 is made up of a single α/β domain that consists of an one <scene name='81/811084/Beta_sheets/6'>β-sheet</scene> with eight parallel β-strands sandwiched between 13 <scene name='81/811084/Alpha_helicesv2/4'>α-helices</scene>. The HDAC8 consists of 377 amino acids. Half of the residues are | + | The HDAC8 is made up of a single α/β domain that consists of an one <scene name='81/811084/Beta_sheets/6'>β-sheet</scene> with eight parallel β-strands sandwiched between 13 <scene name='81/811084/Alpha_helicesv2/4'>α-helices</scene>. The HDAC8 consists of 377 amino acids. Half of the residues are contained in the secondary structure elements while the other half are contained in loops that link the various elements of the secondary structure. The residues forming active site and catalytic machinery of the enzyme is found in the loops from the C-terminal ends of the strands of the core β-sheet. <ref name="Somoza"> Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> |
===Zinc Ion=== | ===Zinc Ion=== | ||
| - | The pentacoordinated Zn<sup>2+</sup> ion involved in the metalloenzyme catalysis is tethered to the protein through interactions with <scene name='81/811085/Zinc_binding/1'>Asp178, His180, and Asp267</scene> . This positions the metal ion to favorably interact with the catalytic water and acetylated lysine substrate. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The | + | The pentacoordinated Zn<sup>2+</sup> ion involved in the metalloenzyme catalysis is tethered to the protein through interactions with <scene name='81/811085/Zinc_binding/1'>Asp178, His180, and Asp267</scene> . This positions the metal ion to favorably interact with the catalytic water and acetylated lysine substrate. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The zinc ion lowers the pKa of a water proton that makes the water more nucleophilic. Additionally, the zinc ion likely also makes the deacetylation process smoother by lowering the entropy of the reaction by binding the nucleophile and the substrate simultaneously, polarizing the carbonyl of the acetyl-lysine and stabilizing the transition state.<ref name="Somoza">Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> |
===Key Residues=== | ===Key Residues=== | ||
| - | The <scene name='81/811085/Active_site/13'>active site</scene> of HDAC8 is composed of 2 catalytic dyads: <scene name='81/811085/Dyads/ | + | The <scene name='81/811085/Active_site/13'>active site</scene> of HDAC8 is composed of 2 catalytic dyads: <scene name='81/811085/Dyads/4'>His143/Asp183 and His142/Asp176</scene>, which activate the catalytic water nucleophile. A Tyr306, through mutation to Phe in the pdb file 2v5w (modeled in the overall view) was observed to render the protein mostly inactive. Thus, it has been hypothesized that the this residue is critical for stabilization of the transition state with the Zn<sup>2+</sup> ion. This mutation allowed the determination of the crystal structure of HDAC8 in complex with the ligand. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> |
===Binding Pocket=== | ===Binding Pocket=== | ||
| Line 37: | Line 37: | ||
==Mechanism== | ==Mechanism== | ||
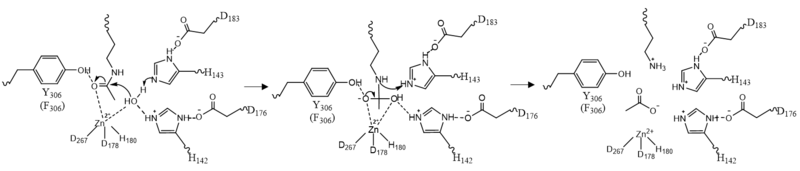
| - | Once the substrate is bound to the binding pocket through interactions with <scene name='81/811087/Ligand_interaction/2'>Asp101, Phe152 and Phe208</scene>, the water molecule attacks the carbonyl carbon of the ε-amino-lysine sidechain of N-terminal core of histone proteins (Figure 2). This water molecule is recruited and stabilized by <scene name='81/811085/Dyads/ | + | Once the substrate is bound to the binding pocket through interactions with <scene name='81/811087/Ligand_interaction/2'>Asp101, Phe152 and Phe208</scene>, the water molecule attacks the carbonyl carbon of the ε-amino-lysine sidechain of N-terminal core of histone proteins (Figure 2). This water molecule is recruited and stabilized by <scene name='81/811085/Dyads/4'>two catalytic dyads</scene>. The first dyad consists of His143 and Asp183. Asp183 interacts with His143 to shift electron density so that His143 may act as a general base to remove a proton from water. The second catalytic dyad consists of His142 and Asp176 and stabilizes the now deprotonated water molecule. A Zn<sup>2+</sup> ion also makes the water more acidic making it a better nucleophile. The tetrahedral intermediate is stabilized by the Zn<sup>2+</sup> ion as well as Tyr306. The amine group of the substrate's lysine acts as a general base and deprotonates His143. This drives the tetrahedral intermediate to collapse and expel the acetyl group to produce an acetate ion and a deacetylated lysine residue. <ref name="Seto, E., & Yoshida, M."> Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713 </ref> |
[[Image:Mech.PNG|800px||center||thumb|Figure 2: HDAC8 Mechanism: Tyr306 was mutated to Phe306 to determine the crystal structure in the pdb file 2v5w.]] | [[Image:Mech.PNG|800px||center||thumb|Figure 2: HDAC8 Mechanism: Tyr306 was mutated to Phe306 to determine the crystal structure in the pdb file 2v5w.]] | ||
Revision as of 13:31, 26 April 2019
Histone Deacetylase 8 (HDAC 8)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047
- ↑ DesJarlais, R., & Tummino, P. J. (2016). Role of histone-modifying enzymes and their complexes in regulation of chromatin biology. Biochemistry, 55(11), 1584-1599. https://doi.org/10.1021/acs.biochem.5b01210
- ↑ 3.0 3.1 3.2 3.3 3.4 Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012
- ↑ Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634. https://doi.org/10.1016/j.bmc.2011.06.030
- ↑ 5.0 5.1 5.2 Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., ... & Steinkühler, C. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences, 101(42), 15064-15069. https://dx.doi.org/10.1073%2Fpnas.0404603101
- ↑ Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713
- ↑ Eckschlager T, Plch, J, Stiborova M, Hrabeta J.Histone deacetylase inhibitors as anticancer drugs. International journal of molecular sciences, 18(7), 1414. 2017. https://dx.doi.org/10.3390%2Fijms18071414