This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Asif Hossain/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
| - | Histone deacetylase 8 (HDAC8) is an enzyme that plays a role in controlling gene expression. Specifically, HDAC8 removes an acetyl group off of the ε-amino-Lys 382 of Histone 4's N-terminal core.<ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> [https://en.wikipedia.org/wiki/Histone Histones] consist of eight monomers to form an octomer complex. Each histone has a positive charge which allows interaction with negatively-charged DNA. This prevents transcription factors from accessing DNA, thus, decreasing gene expression. [https://en.wikipedia.org/wiki/Chromatin_remodeling Chromatin remodeling] by | + | Histone deacetylase 8 (HDAC8) is an enzyme that plays a role in controlling gene expression. Specifically, HDAC8 removes an acetyl group off of the ε-amino-Lys 382 of Histone 4's N-terminal core.<ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> [https://en.wikipedia.org/wiki/Histone Histones] consist of eight monomers to form an octomer complex. Each histone has a positive charge which allows interaction with negatively-charged DNA. This prevents transcription factors from accessing DNA, thus, decreasing gene expression. [https://en.wikipedia.org/wiki/Chromatin_remodeling Chromatin remodeling] by histone acetylation and/or deacetylation is an example of [https://en.wikipedia.org/wiki/Epigenetics epigenetic regulation]. [https://en.wikipedia.org/wiki/Histone_acetyltransferase Histone Acetylase 1] (HAT1) catalyzes the addition of an acetyl group onto a histone. The lack of charge on the acetyl group weakens the interaction between DNA and histones which allows transcription factors to access the DNA to increase gene expression. HDAC8 reverses this reaction by catalyzing the removal of these acetyl groups from the Lys to reclaim the positive charge of the histone. This allows the histone to interact with the negative charge on the DNA. As a result, DNA binds more tightly to the histone protein, repressing transcription and gene expression. |
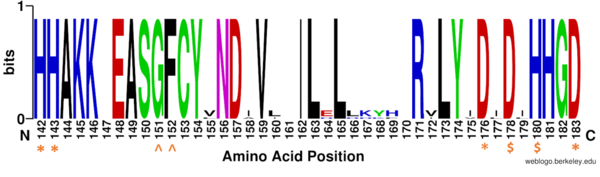
==HDAC Enzymes and Homology== | ==HDAC Enzymes and Homology== | ||
| Line 16: | Line 16: | ||
===Zinc Ion=== | ===Zinc Ion=== | ||
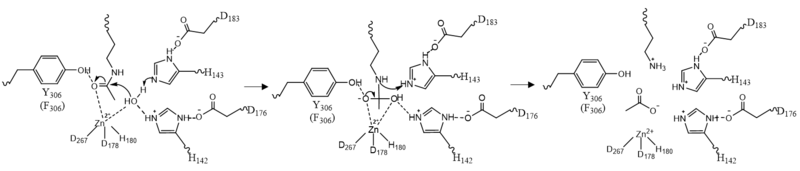
| - | The pentacoordinated Zn<sup>2+</sup> ion involved in the metalloenzyme catalysis is tethered to the protein through interactions with <scene name='81/811085/Zinc_binding/1'>Asp178, His180, and Asp267</scene> . This positions the metal ion to favorably interact with the catalytic water and acetylated lysine substrate. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The Zn<sup>2+</sup> ion lowers the pKa of a water molecule that activates it as a nucleophile. | + | The pentacoordinated Zn<sup>2+</sup> ion involved in the metalloenzyme catalysis is tethered to the protein through interactions with <scene name='81/811085/Zinc_binding/1'>Asp178, His180, and Asp267</scene> . This positions the metal ion to favorably interact with the catalytic water and acetylated lysine substrate. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The Zn<sup>2+</sup> ion lowers the pKa of a water molecule that activates it as a nucleophile. Additionally, the Zn<sup>2+</sup> ion likely also makes the deacetylation process smoother by lowering the entropy of the reaction by binding the nucleophile and the substrate simultaneously, polarizing the carbonyl of the acetyl-lysine and stabilizing the transition state.<ref name="Somoza">Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> |
===Key Residues=== | ===Key Residues=== | ||
| Line 22: | Line 22: | ||
===Binding Pocket=== | ===Binding Pocket=== | ||
| - | By encasing the nonpolar, four-carbon side-chain of the Lys residue on the ligand, Phe152 and Phe208 engage in hydrophobic Van der Waals interactions with the ligand at different ends of the <scene name='81/811085/Binding_pocket_surface/3'>binding pocket</scene>. Trp141 and Met274 contribute to the overall shape through general hydrophobic interactions.<ref name="Whitehead">Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634. https://doi.org/10.1016/j.bmc.2011.06.030 </ref> Finally, the carbonyl oxygen of <scene name='81/811085/Binding_pocket_glycine/ | + | By encasing the nonpolar, four-carbon side-chain of the Lys residue on the ligand, Phe152 and Phe208 engage in hydrophobic Van der Waals interactions with the ligand at different ends of the <scene name='81/811085/Binding_pocket_surface/3'>binding pocket</scene>. Trp141 and Met274 contribute to the overall shape through general hydrophobic interactions.<ref name="Whitehead">Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634. https://doi.org/10.1016/j.bmc.2011.06.030 </ref> Finally, the carbonyl oxygen of <scene name='81/811085/Binding_pocket_glycine/2'>Gly151</scene> hydrogen bonds with the amide hydrogen of the acetylated lysine to further interact with the ligand in the relatively hydrophobic tunnel.<ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> |
At the rim of the active site, <scene name='81/811084/Asp101/5'>Asp101</scene> is involved in two hydrogen bonds between its own carbonyl oxygens and two consecutive amide hydrogens of incoming <scene name='81/811084/Ligand/8'>peptide derived ligand</scene>. This forces the ligand to assume a cis-conformation. In addition, extensive interactions among many other polar atoms near the rim of the active site help keep the ligand lodged in the hydrophobic tunnel.<ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> | At the rim of the active site, <scene name='81/811084/Asp101/5'>Asp101</scene> is involved in two hydrogen bonds between its own carbonyl oxygens and two consecutive amide hydrogens of incoming <scene name='81/811084/Ligand/8'>peptide derived ligand</scene>. This forces the ligand to assume a cis-conformation. In addition, extensive interactions among many other polar atoms near the rim of the active site help keep the ligand lodged in the hydrophobic tunnel.<ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> | ||
===Additional Features=== | ===Additional Features=== | ||
| - | There are two | + | There are two potassium ions bound in the HDAC8 structure. Potassium 1 is 7Å away from the active site while potassium 2 lies toward the exterior of the HDAC8.<ref name="Vannini, A., Volpari, C., Filocamo, G.">Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., ... & Steinkühler, C. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences, 101(42), 15064-15069. https://dx.doi.org/10.1073%2Fpnas.0404603101</ref>. It is suggested that potassium 1 is of interest to the active site of HDAC8 because it is tethered to the enzyme by the main chain carbonyl oxygens of Asp178 and His180 which stabilizes the Zn<sup>2+</sup> in the active site. Furthermore, the potassium ion increases the positive charge in the active site and this could help stabilize the oxyanion hole that is formed in the transition state.<ref name="Vannini, A., Volpari, C., Filocamo, G.">Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., ... & Steinkühler, C. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences, 101(42), 15064-15069. https://dx.doi.org/10.1073%2Fpnas.0404603101</ref> |
<scene name='81/811087/Active_site_loop_1_s30-k36/11'>N-Terminus L1 loop </scene>(amino acid residues 30-36) makes up a significant part of the active site pocket. It is suggested that this loop has high flexibility that enables HDAC8 to more efficiently adjust binding to different ligands. <ref name="Somoza">Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> | <scene name='81/811087/Active_site_loop_1_s30-k36/11'>N-Terminus L1 loop </scene>(amino acid residues 30-36) makes up a significant part of the active site pocket. It is suggested that this loop has high flexibility that enables HDAC8 to more efficiently adjust binding to different ligands. <ref name="Somoza">Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> | ||
Revision as of 14:41, 26 April 2019
Histone Deacetylase 8 (HDAC 8)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047
- ↑ DesJarlais, R., & Tummino, P. J. (2016). Role of histone-modifying enzymes and their complexes in regulation of chromatin biology. Biochemistry, 55(11), 1584-1599. https://doi.org/10.1021/acs.biochem.5b01210
- ↑ 3.0 3.1 3.2 3.3 3.4 Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012
- ↑ Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634. https://doi.org/10.1016/j.bmc.2011.06.030
- ↑ 5.0 5.1 5.2 Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., ... & Steinkühler, C. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences, 101(42), 15064-15069. https://dx.doi.org/10.1073%2Fpnas.0404603101
- ↑ Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713
- ↑ 7.0 7.1 Eckschlager T, Plch, J, Stiborova M, Hrabeta J.Histone deacetylase inhibitors as anticancer drugs. International journal of molecular sciences, 18(7), 1414. 2017. https://dx.doi.org/10.3390%2Fijms18071414