We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Emily Leiderman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
=== Hydrophobic Pocket === | === Hydrophobic Pocket === | ||
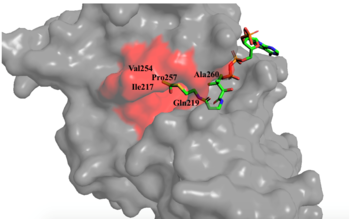
[[Image:hydroophobic pocket.png|350px|right|thumb|Figure 1. Image of the substrate Acetyl CoA bound in the groove of HAT1 (1BOB.pdb). The groove contains the three residues involved in the acetyltransferase mechanism.]] The active site consists of the Acetyl CoA ligand bound to the enzyme in a groove on the surface of the protein. The [https://en.wikipedia.org/wiki/Ligand ligand] is held in place by several bonds to protein residues that result in the formation of a <scene name='81/811098/Hydrophobic_pocket/2'>hydrophobic pocket</scene>. The hydrophobic pocket consists of the interacting side chains from residues <scene name='81/811098/Ile-217_pro-257_phe-261/3'>Ile-217, Pro-257, and Phe-261</scene> in addition to further bonds resulting from residues 217-220 and 255-256 <ref name=Dut/> (figure 1). The amide of main-chain <scene name='81/811098/Phe-220/4'>Phe-220</scene> hydrogen bonds to the carbonyl oxygen of the Acetyl group in the binding pocket. The main-chain amide of <scene name='81/811098/Asn-258/4'>Asn-258</scene> also donates a hydrogen bond from its side chain to oxygen PO5 of the pantothenic acid group <ref name=Dut/>. The binding within the hydrophobic pocket is further supplemented through the creation of a protein gate that establishes a bridge over the concave surface that serves to keep Acetyl CoA in place while the enzyme interacts with the histone. | [[Image:hydroophobic pocket.png|350px|right|thumb|Figure 1. Image of the substrate Acetyl CoA bound in the groove of HAT1 (1BOB.pdb). The groove contains the three residues involved in the acetyltransferase mechanism.]] The active site consists of the Acetyl CoA ligand bound to the enzyme in a groove on the surface of the protein. The [https://en.wikipedia.org/wiki/Ligand ligand] is held in place by several bonds to protein residues that result in the formation of a <scene name='81/811098/Hydrophobic_pocket/2'>hydrophobic pocket</scene>. The hydrophobic pocket consists of the interacting side chains from residues <scene name='81/811098/Ile-217_pro-257_phe-261/3'>Ile-217, Pro-257, and Phe-261</scene> in addition to further bonds resulting from residues 217-220 and 255-256 <ref name=Dut/> (figure 1). The amide of main-chain <scene name='81/811098/Phe-220/4'>Phe-220</scene> hydrogen bonds to the carbonyl oxygen of the Acetyl group in the binding pocket. The main-chain amide of <scene name='81/811098/Asn-258/4'>Asn-258</scene> also donates a hydrogen bond from its side chain to oxygen PO5 of the pantothenic acid group <ref name=Dut/>. The binding within the hydrophobic pocket is further supplemented through the creation of a protein gate that establishes a bridge over the concave surface that serves to keep Acetyl CoA in place while the enzyme interacts with the histone. | ||
| - | |||
| - | |||
| Line 32: | Line 30: | ||
== Mechanism == | == Mechanism == | ||
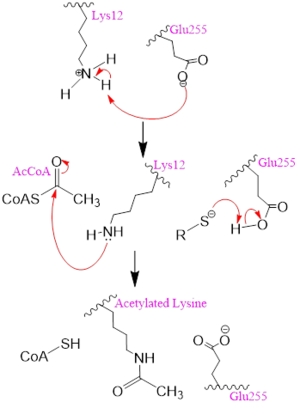
| - | [[Image: | + | [[Image:mechh.PNG|300px|left|thumb|Figure 2. A possible acetylation mechanism of HAT1 (PDB: 1BOB). Mechanism consists of deptronation of Lys-12 of histone H4 and then transfer of an acetyl group from Acetyl CoA to Lys-12.]] Several mechanisms for the transfer of the acetyl group to the Lys-12 of the histone have been proposed. The most accurate and likely mechanism today is conserved across several HAT1 studies and consists of deprotonating the amino group on the Lys-12 of H4 (figure 2). This mechanism was proposed by Wu et al with respect to the human HAT1 protein. Analysis was done on the 1BOB model (retrieved from the protein database) and similar residues with respect to location and function were identified. The main chain carbonyl oxygen of the polar residues <scene name='81/811098/Gln-219_glu-255_asp-256/3'>Gln-219, Glu-255, and Asp-256</scene> are predicted to deprotonate the Lys-12 reside of H4 as seen in figure 2 <ref name=Wu>PMID:22615379 </ref>. Structural analysis of the 4PSW protein shows that the main chain carbonyls of these three residues are found in close proximity of the amino group of Lys-12 <ref name=idk>PMID: 24835250 </ref>. Of those residues it is uncertain which (if any) accept the proton of Lys-12. Each main chain carbonyl oxygen belonging to these three residues falls within 2 to 3 angstroms of the target amide on Lysine 12. Deprotonation of the amino group on the Lys-12 makes the residue nucleophilic enough to directly attack the carbonyl carbon of the acetyl group to initiate the acetyl transfer. We selected Glu255 to be the proton acceptor in our proposed mechanism displayed in figure 2. The transfer mechanism is contingent on the conformational change and the formation of a functional gate that spans the concave groove over the bound Acetyl CoA. This gate holds the substrate in place while the enzymatic [https://en.wikipedia.org/wiki/Deprotonation deprotonation] process takes place <ref name=Wu/>. |
Revision as of 17:20, 26 April 2019
Histone Acetyltransferase 1
| |||||||||||