This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Asif Hossain/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
===Zinc Ion=== | ===Zinc Ion=== | ||
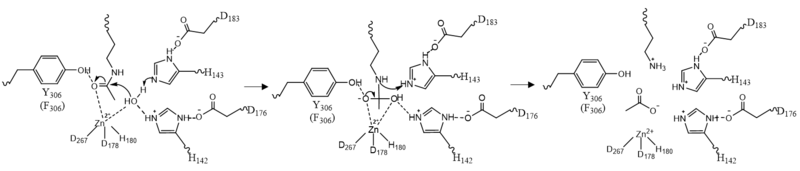
| - | The pentacoordinated Zn<sup>2+</sup> ion involved in the metalloenzyme catalysis is tethered to the protein through interactions with <scene name='81/811085/Zinc_binding/2'>Asp178, His180, and Asp267</scene> . This positions the metal ion to favorably interact with the catalytic water and acetylated lysine substrate. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The Zn<sup>2+</sup> ion lowers the pKa of a water molecule that activates it as a nucleophile. By binding to both the nucleophile and the substrate simultaneously, the Zn<sup>2+</sup> ion also assists the deacetylation process by lowering the entropy of the reaction. Thus, the the Zn<sup>2+</sup> polarizes the carbonyl of the acetyl-lysine and stabilizes the transition state.<ref name="Somoza">Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> | + | The pentacoordinated Zn<sup>2+</sup> ion involved in the metalloenzyme catalysis is tethered to the protein through interactions with <scene name='81/811085/Zinc_binding/2'>Asp178, His180, and Asp267</scene>. This positions the metal ion to favorably interact with the catalytic water and acetylated lysine substrate. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The Zn<sup>2+</sup> ion lowers the pKa of a water molecule that activates it as a nucleophile. By binding to both the nucleophile and the substrate simultaneously, the Zn<sup>2+</sup> ion also assists the deacetylation process by lowering the entropy of the reaction. Thus, the the Zn<sup>2+</sup> polarizes the carbonyl of the acetyl-lysine and stabilizes the transition state.<ref name="Somoza">Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> |
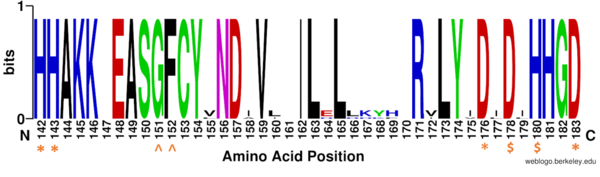
===Key Residues=== | ===Key Residues=== | ||
Revision as of 17:55, 26 April 2019
Histone Deacetylase 8 (HDAC 8)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047
- ↑ DesJarlais, R., & Tummino, P. J. (2016). Role of histone-modifying enzymes and their complexes in regulation of chromatin biology. Biochemistry, 55(11), 1584-1599. https://doi.org/10.1021/acs.biochem.5b01210
- ↑ 3.0 3.1 3.2 3.3 3.4 Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012
- ↑ Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634. https://doi.org/10.1016/j.bmc.2011.06.030
- ↑ 5.0 5.1 5.2 Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., ... & Steinkühler, C. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences, 101(42), 15064-15069. https://dx.doi.org/10.1073%2Fpnas.0404603101
- ↑ Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713
- ↑ 7.0 7.1 Eckschlager T, Plch, J, Stiborova M, Hrabeta J.Histone deacetylase inhibitors as anticancer drugs. International journal of molecular sciences, 18(7), 1414. 2017. https://dx.doi.org/10.3390%2Fijms18071414
Contributors
Asif Hossain, Sean O'Brien, and Josephine Thestrup