Cystathionine β-synthase
From Proteopedia
(Difference between revisions)
(New page: ==3D Structure of Human Cystathionine β-synthase (4coo)== <StructureSection load='4coo' size='340' side='right' caption='Human Cystathionine β-synthase' scene=''> Cystathionine β-syntha...) |
|||
| Line 15: | Line 15: | ||
The central part of the dimer interface is formed by the residues Phe111 and Phe112 close to the 2-fold dimer axis, thus Phe112 of monomer A interacts with Phe112 of monomer B and vice versa. Polar interactions also contribute to the dimer interactions. On the other hand, the guanidium group of Arg379 is completely buried within the core of the dimer interface and rather than involved in any polar interactions between monomers forms hydrogen bonds to Gly115 and Asn380 of the same monomer.<ref>PMID:11483494</ref> | The central part of the dimer interface is formed by the residues Phe111 and Phe112 close to the 2-fold dimer axis, thus Phe112 of monomer A interacts with Phe112 of monomer B and vice versa. Polar interactions also contribute to the dimer interactions. On the other hand, the guanidium group of Arg379 is completely buried within the core of the dimer interface and rather than involved in any polar interactions between monomers forms hydrogen bonds to Gly115 and Asn380 of the same monomer.<ref>PMID:11483494</ref> | ||
| + | '''The active site''' | ||
| - | + | The coenzyme PLP is deeply buried in a cleft between the N- and C-terminal domains, and the active site is accessible only via a narrow channel. The cofactor is linked to the ε-amino group of Lys119 via a Schiff base linkage forming the so-called 'internal aldimine'. The nitrogen of the pyridine ring forms a hydrogen bond to the Oγ of Ser349. Another hydrogen bond is formed between the 3’ hydroxyl group of PLP and the Nδ2 of Asn149. This residue is coplanar with the pyridine ring and thus allows the expected ring tilt upon transaldimination. | |
| + | The phosphate binding loop is located between β-strand 8 and α-helix 8 and is composed of the residues Gly256, Thr257, Gly258, Gly259 and Thr260. These residues form an extended hydrogen bonding network with the phosphate moiety of PLP, thus anchoring the cofactor to the protein matrix. In addition, the positive pole of the helix dipole from α-helix 8 compensates for the negative charge of the phosphate group. | ||
| + | Residues Tyr223 and Gly307 are probably the key residues for substrate specificity, as they are spatially adjacent to the substrate binding site. After binding substrate the catalytic core undergoes a conformational change.<ref>PMID:11483494</ref> | ||
| - | + | '''The heme binding site''' | |
| - | + | Heme molecules are located at distal ends of the dimers with the orientation of their ring planes normal to the protein surface. The heme is bound in a hydrophobic pocket formed by residues 50±67, α-helices 6 and 8 and a loop preceding β-strand 10. The sulfhydryl group of Cys52 and the Nε2 atom of His65 axially coordinate the iron in the heme. The sulfur atom of Cys52 is deprotonated and forms additional polar interactions with the side chain of Arg266 and the main chain nitrogen of Trp54. Nε2 of His65 is solvent accessible and lacking any hydrogen bonding partner from protein residues. | |
| + | The heme carboxylate groups are involved in ionic interactions with Arg51 and Arg224 and are partially solvent accessible. | ||
| + | Since the iron ion is ligated from both sides by protein residues this makes an enzymatic role of the heme unlikely. There is no covalent attachment of the heme to the protein which means it can be reversibly released under reducing conditions in the presence of carbon monoxide (CO). Under oxidizing conditions, the heme cannot be released, probably because CO does not bind to heme in its ferric state. | ||
| + | It is likely that CO displaces one of the axial heme ligands, followed by a local unfolding of the N-terminal residues leading to a release of the prosthetic group. The displaced ligand is probably the cysteine, because the absorption spectrum of CBS treated with CO is similar to the spectra of other CO±heme±imidazole protein complexes.<ref>PMID:11483494</ref> | ||
| - | + | '''Oxidoreductase active site motif''' | |
| + | |||
| + | The loop between α-helix 8 and β-strand 9 harbors a motif similar to the active site of disulfide oxidoreductases. In CBS this motif consists of the sequence CPGC (residues 272±275) and forms a β-turn. The two cysteines are oxidized and form a disulfide bridge. The disulfide bridge is in a right-handed hook conformation and is located on the surface of the protein and hence is solvent accessible. The same two cysteines, however, are not solvent accessible in the full-length enzyme.<ref>PMID:11483494</ref> | ||
| + | |||
| + | '''The regulatory domain''' | ||
| + | |||
| + | Full-length CBS contains a C-terminal regulatory domain of ~140 residues, including two so-called 'CBS domains' (CBS1 of 53 residues and CBS2 of 57 residues). The C-terminal domain of CBS contains an autoinhibitory region that gets displaced from the active site upon binding of the allosteric activator AdoMet. | ||
| + | The fact that truncated CBS forms dimers rather than tetramers or higher order oligomers suggests that the regulatory domain is involved in tetramer formation. | ||
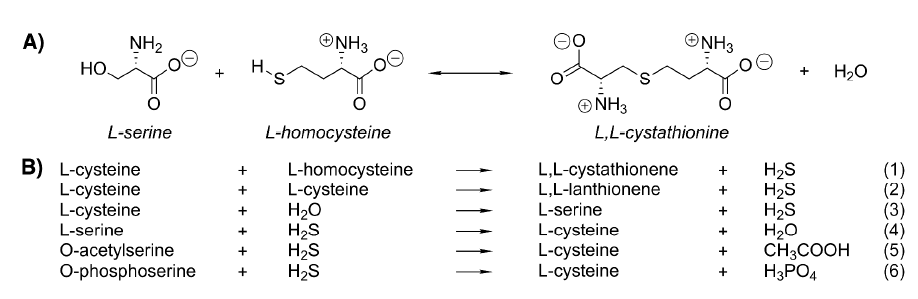
| + | [[Image:Reactions catalyzed by CBS.png]] | ||
| + | |||
| + | == Disease == | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 19:38, 28 April 2019
3D Structure of Human Cystathionine β-synthase (4coo)
| |||||||||||
References
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910