We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Cystathionine β-synthase
From Proteopedia
(Difference between revisions)
| Line 36: | Line 36: | ||
Full-length CBS contains a C-terminal regulatory domain of ~140 residues, including two so-called 'CBS domains' (CBS1 of 53 residues and CBS2 of 57 residues). The C-terminal domain of CBS contains an autoinhibitory region that gets displaced from the active site upon binding of the allosteric activator AdoMet. | Full-length CBS contains a C-terminal regulatory domain of ~140 residues, including two so-called 'CBS domains' (CBS1 of 53 residues and CBS2 of 57 residues). The C-terminal domain of CBS contains an autoinhibitory region that gets displaced from the active site upon binding of the allosteric activator AdoMet. | ||
The fact that truncated CBS forms dimers rather than tetramers or higher order oligomers suggests that the regulatory domain is involved in tetramer formation. | The fact that truncated CBS forms dimers rather than tetramers or higher order oligomers suggests that the regulatory domain is involved in tetramer formation. | ||
| + | |||
| + | == The Reaction Catalyzed by CBS == | ||
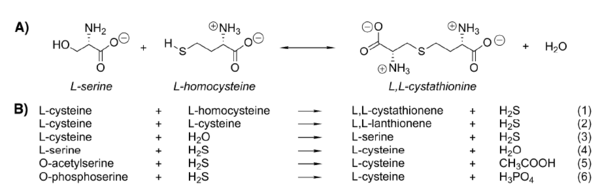
| + | Cystathionine β-synthase catalyzes β-elimination and β-replacement reactions. In a typical situation it condensates L-serine and L-homocysteine to give cystathionine but there are also other possible substrates for this enzyme leading to different products. | ||
| + | The reaction for synthesis of cystathionine starts with displacement of the internal aldimine between the enzyme active site lysine and pyridoxal 5’-phosphate (E-PLP) by the incoming L-serine. The serine external aldimine adduct (E-PLP-L-ser) forms an aminoacrylate intermediate (E-PLP-aa) that reacts with the incoming second substrate, such as L-homocysteine, to form the (L,L)-cystathionine external aldimine, which is then displaced by the active site lysine to regenerate the active enzyme (7). | ||
| + | The type of reaction mechanisms used by the CBS is known as a double displacement or ping-pong mechanism. The rate determining step in the reaction is hydrolysis of the external aldimine of cystathionine (5). | ||
[[Image:Reactions catalyzed by CBS.png|600px|left Reactions catalyzed by CBS. (A) Canonical reaction of CBS. (B) CBS reactions that generate or utilize H2S.]] | [[Image:Reactions catalyzed by CBS.png|600px|left Reactions catalyzed by CBS. (A) Canonical reaction of CBS. (B) CBS reactions that generate or utilize H2S.]] | ||
| + | |||
== Disease == | == Disease == | ||
Revision as of 19:43, 28 April 2019
3D Structure of Human Cystathionine β-synthase (4coo)
| |||||||||||
References
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910