Cystathionine β-synthase (CBS; EC 4.2.1.22) is a unique pyridoxal 5’-phosphate (PLP) enzyme which plays a crucial role in the transsulfuration metabolic pathway of sulfur-containing amino acid, L-methionine.
PLP enzymes are divided into four families on the basis of similarities in three dimensional structure, sequence, secondary structure, and hydrophobicity profiles. CBS is a member of the β family or Fold type II, which also contains O-acetylserine sulfhydrylase (OASS) and O-acetyl-L-serine(thiol)lyase (OASTL)[1].
Structure of CBS
The human form of CBS is a homotetramer consisting of 63-kDa subunits each of 551 amino acids in length. Two cofactors, pyridoxal 5’-phosphate (PLP) and heme, and two substrates, L-homocysteine and L-serine, are bound to every monomer and the function is further allosterically regulated by S-adenosyl-L-methionine (AdoMet).
The monomer has a complex domain structure as it is composed of three functional domains, two of which are regulatory and one is catalytic. The middle domain contains the catalytic core responsible for the pyridoxal 5’-phosphate-catalyzed reaction and is flanked by an N-terminal heme-binding domain (70 amino acids), which probably regulates the enzyme in response to redox conditions, and a C-terminal Bateman module consisting of two CBS domains (CBS1, CBS2), protein folding motifs also found in inosine 5’-monophosphate dehydrogenase, chloride channels and several other proteins in various organisms.[2] The C-terminal domain also contains a negative regulatory region that is responsible for allosteric activation of the enzyme by AdoMet.
Truncation of N-terminal domain produces an enzyme that is still active albeit less so (19%) than the wild type. Truncation of C-terminal domain activates the enzyme by ~2-fold over full-length wild type. In addition, deletion of the Bateman module leads to a change in oligomerization from a tetrameric to a dimeric form of the enzyme (4,7).
The CBS tetramer of the full-length enzyme has a strong tendency to aggregate, which makes physical studies very difficult. Therefore recombinant human CBS comprising the amino acid residues 1±413 (C-terminal domain missing), which does not exhibit the aggregating properties, is described.
The monomer is composed of 11 α-helices, seven short 310 helices and two β-sheets consisting of four (in the N-terminal domain) and six strands (in the C-terminal domain), respectively. The additional β-strand 1 interacts with strand 2 of the C-terminal sheet of the other monomer in the dimer in a parallel manner. The heme binding motif lacks any secondary structure with the exception of a short 310 helix.
The dimer interface is mainly hydrophobic in character and is composed of the side chains of the residues Ile76, Leu77, Ile80, Thr87, Val90, Ile92, Ile152, Leu156, Val160, Val180, Ala183, Leu184, Ile339, Ala340, Leu344, Leu345, Val378, Met382 and Leu386.
The central part of the dimer interface is formed by the residues Phe111 and Phe112 close to the 2-fold dimer axis, thus Phe112 of monomer A interacts with Phe112 of monomer B and vice versa. Polar interactions also contribute to the dimer interactions. On the other hand, the guanidium group of Arg379 is completely buried within the core of the dimer interface and rather than involved in any polar interactions between monomers forms hydrogen bonds to Gly115 and Asn380 of the same monomer.[3]
The active site
The coenzyme PLP is deeply buried in a cleft between the N- and C-terminal domains, and the active site is accessible only via a narrow channel. The cofactor is linked to the ε-amino group of Lys119 via a Schiff base linkage forming the so-called 'internal aldimine'. The nitrogen of the pyridine ring forms a hydrogen bond to the Oγ of Ser349. Another hydrogen bond is formed between the 3’ hydroxyl group of PLP and the Nδ2 of Asn149. This residue is coplanar with the pyridine ring and thus allows the expected ring tilt upon transaldimination.
The phosphate binding loop is located between β-strand 8 and α-helix 8 and is composed of the residues Gly256, Thr257, Gly258, Gly259 and Thr260. These residues form an extended hydrogen bonding network with the phosphate moiety of PLP, thus anchoring the cofactor to the protein matrix. In addition, the positive pole of the helix dipole from α-helix 8 compensates for the negative charge of the phosphate group.
Residues Tyr223 and Gly307 are probably the key residues for substrate specificity, as they are spatially adjacent to the substrate binding site. After binding substrate the catalytic core undergoes a conformational change.[4]
The heme binding site
Heme molecules are located at distal ends of the dimers with the orientation of their ring planes normal to the protein surface. The heme is bound in a hydrophobic pocket formed by residues 50±67, α-helices 6 and 8 and a loop preceding β-strand 10. The sulfhydryl group of Cys52 and the Nε2 atom of His65 axially coordinate the iron in the heme. The sulfur atom of Cys52 is deprotonated and forms additional polar interactions with the side chain of Arg266 and the main chain nitrogen of Trp54. Nε2 of His65 is solvent accessible and lacking any hydrogen bonding partner from protein residues.
The heme carboxylate groups are involved in ionic interactions with Arg51 and Arg224 and are partially solvent accessible.
Since the iron ion is ligated from both sides by protein residues this makes an enzymatic role of the heme unlikely. There is no covalent attachment of the heme to the protein which means it can be reversibly released under reducing conditions in the presence of carbon monoxide (CO). Under oxidizing conditions, the heme cannot be released, probably because CO does not bind to heme in its ferric state.
It is likely that CO displaces one of the axial heme ligands, followed by a local unfolding of the N-terminal residues leading to a release of the prosthetic group. The displaced ligand is probably the cysteine, because the absorption spectrum of CBS treated with CO is similar to the spectra of other CO±heme±imidazole protein complexes.[5]
Oxidoreductase active site motif
The loop between α-helix 8 and β-strand 9 harbors a motif similar to the active site of disulfide oxidoreductases. In CBS this motif consists of the sequence CPGC (residues 272±275) and forms a β-turn. The two cysteines are oxidized and form a disulfide bridge. The disulfide bridge is in a right-handed hook conformation and is located on the surface of the protein and hence is solvent accessible. The same two cysteines, however, are not solvent accessible in the full-length enzyme.[6]
The regulatory domain
Full-length CBS contains a C-terminal regulatory domain of ~140 residues, including two so-called 'CBS domains' (CBS1 of 53 residues and CBS2 of 57 residues). The C-terminal domain of CBS contains an autoinhibitory region that gets displaced from the active site upon binding of the allosteric activator AdoMet.
The fact that truncated CBS forms dimers rather than tetramers or higher order oligomers suggests that the regulatory domain is involved in tetramer formation.
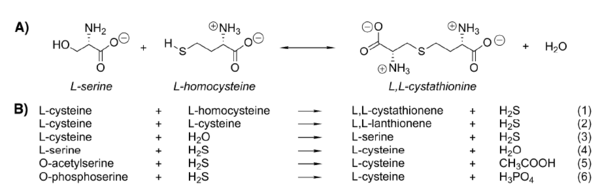
The Reaction Catalyzed by CBS
Cystathionine β-synthase catalyzes β-elimination and β-replacement reactions. In a typical situation it condensates L-serine and L-homocysteine to give cystathionine but there are also other possible substrates for this enzyme leading to different products.
The reaction for synthesis of cystathionine starts with displacement of the internal aldimine between the enzyme active site lysine and pyridoxal 5’-phosphate (E-PLP) by the incoming L-serine. The serine external aldimine adduct (E-PLP-L-ser) forms an aminoacrylate intermediate (E-PLP-aa) that reacts with the incoming second substrate, such as L-homocysteine, to form the (L,L)-cystathionine external aldimine, which is then displaced by the active site lysine to regenerate the active enzyme (7).
The type of reaction mechanisms used by the CBS is known as a double displacement or ping-pong mechanism. The rate determining step in the reaction is hydrolysis of the external aldimine of cystathionine (5).

Disease


