User:Alice Kei Endo/Sandbox 1
From Proteopedia
| Line 2: | Line 2: | ||
<Structure load='1PUO' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='1PUO' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
| + | |||
| + | == Function == | ||
| + | It has been suggested that its role is to protect the skin, by homology with the uteroglobin whose function is to protect mucosa. Other authors believe that Fel d 1 would rather have a role in the transport of lipid molecules, especially steroids, hormones or pheromones. | ||
== Disease == | == Disease == | ||
| Line 34: | Line 37: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | Karn RC. The mouse salivary androgen-binding protein (ABP) alpha subunit closely resembles chain 1 of the cat allergen Fel dI. Biochem Genet. 1994;32:271–277. | ||
| + | |||
| + | Vervloet D, Birnbaum J. Origine des allergènes du chat. Rev Fr Allergol. 1995;35:533–538. | ||
| + | |||
| + | Kaiser L, Velickovic TC, Badia-Martinez D, Adedoyin J, Thunberg S, Hallén D, et al. Structural characterization of the tetrameric form of the major cat allergen Fel d 1. J Mol Biol. 2007;370:714–727. | ||
<references/> | <references/> | ||
Revision as of 21:26, 16 June 2019
Contents |
Fel d 1
|
Function
It has been suggested that its role is to protect the skin, by homology with the uteroglobin whose function is to protect mucosa. Other authors believe that Fel d 1 would rather have a role in the transport of lipid molecules, especially steroids, hormones or pheromones.
Disease
Structural highlights
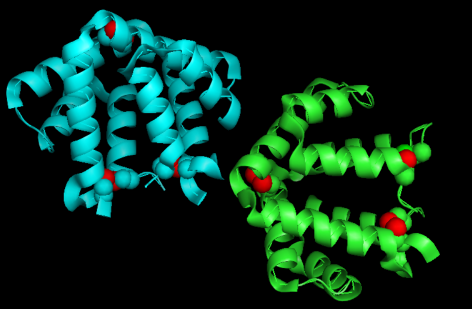
Fel d 1 has been reported as a 35 kDa tetrameric glycoprotein formed by two heterodimers, consisting of named H1–H4 (corresponding to chain 1) and H5–H8 (corresponding to chain 2). Each heterodimer consists of one 70 residue peptide and one 85, 90, or 92 residue peptide (i.e. chains 1 and 2, respectively) encoded by separate genes. These chains are linked within each heterodimer through three disulfide bonds, formed between cysteine residues Cys3 and Cys73, Cys44 and Cys48, and Cys70 and Cys7, in chains 1 and 2, respectively.
In the image below, we can see the 3 disulfide bonds in red:
Crystal structure
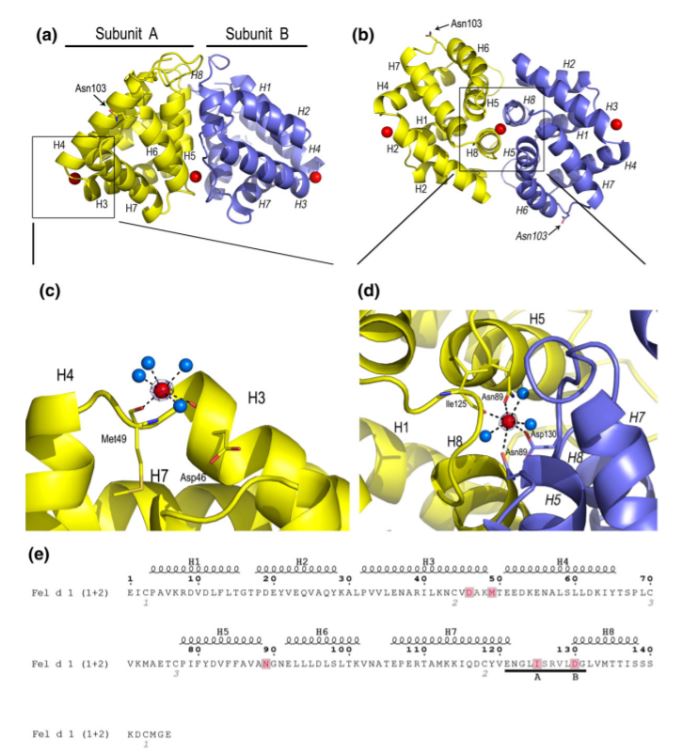
The crystal structure of Fel d 1 tetramer, at 1.6 Å resolution, displays a globular all helical fold that composed of having four Ca ions, 270 water molecules, a and a . As said before each subunit of the Fel d 1 consists of eight helices named H1–H4 (corresponding to chain 1) and H5–H8 (corresponding to chain 2).
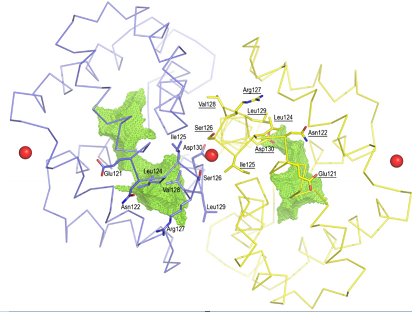
A total of four Ca ions are present in the crystal structure of the Fel d 1. One Ca2+ (site 1) is bound within the dimerization interface, resulting in profound local conformational changes. The binding of the Ca2+ in this region of the Fel d 1 interface most probably stabilizes the tetrameric form, since the presence of such a positively charged ion allows for the packing of acidic residues ( Asp130 in subunits A and B) in the Fel d 1 tetramer interface. However, at the same time it also results in profound local conformational changes that affect the size and form of the different cavities. Two additional Ca2+-binding sites are also present on both sides of the tetramer, localized at exactly the same position as suggested for Ca2+ binding sites in uteroglobin, one on each of the heterodimeric subunits A and B. At last Ca2+ site has been shown, however the position of this ion is most probably due to the packing of the molecules in the asymmetric unit.
In the image below, the CA2+ ions are represented in red. In (A) we can see two Ca2+-binding site localized at exactly the same position as suggested for Ca2+ binding sites in uteroglobin. (B) Ca2+ bound within the dimerization interface. (C) amplified vision of the Ca2+-binding site localized at exactly the same position as suggested for Ca2+ binding sites in uteroglobin. (D) amplified Ca2+ bound within the dimerization interface.
The crystal structure of Fel d 1 also suggests a potential portal for two waterfilled cavities of different size, one in each monomer.
The binding of a Ca2+ in the dimerization interface results in a profound local conformational change
</StructureSection>
References
Karn RC. The mouse salivary androgen-binding protein (ABP) alpha subunit closely resembles chain 1 of the cat allergen Fel dI. Biochem Genet. 1994;32:271–277.
Vervloet D, Birnbaum J. Origine des allergènes du chat. Rev Fr Allergol. 1995;35:533–538.
Kaiser L, Velickovic TC, Badia-Martinez D, Adedoyin J, Thunberg S, Hallén D, et al. Structural characterization of the tetrameric form of the major cat allergen Fel d 1. J Mol Biol. 2007;370:714–727.