User:Luis Andres Casavilca Ramirez/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
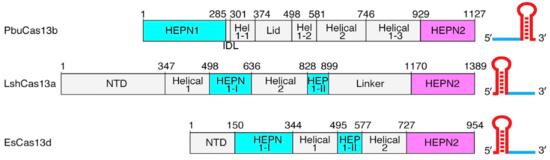
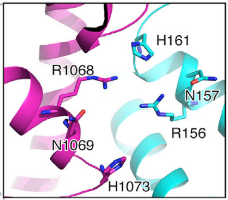
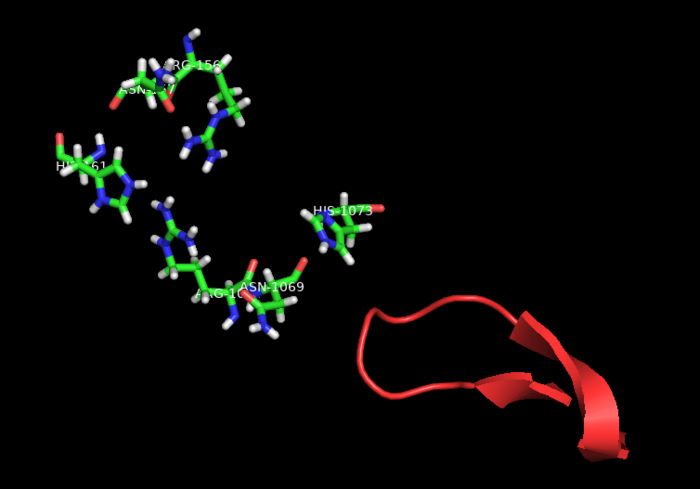
HEPNs (Higher Eukaryotes and Prokaryotes Nucleotide-binding domain) are alpha helical domains, many of which are present in RNA maturation systems and related biological conflicts and most of which contain a catalytic Rx4-6H active site. Cas 13b <scene name='81/817991/Hepn1/1'>HEPN1</scene> domain is composed of 12 linearly connected alpha helices flexible loop between them, whereas <scene name='81/817991/Hepn2/1'>HEPN2</scene> contains nine alpha helices, several short beta strands and beta-hairpin with positively charged residues at the tip. In accordance with the conserved active site from HEPNs, a Rx4-6H site is present, where active residues locate within both HEPN1 (<scene name='81/817991/Hepn1_active_site/1'>R156, N157, H161</scene>) and HEPN2 (<scene name='81/817991/Hepn2_active_site/1'>R1068, N1069, H1073</scene>) domains. One of the two histidines is thought to act as a base, inducing the ribose 2’OH to attack the phosphodiester linkage. The conserved arginine could stabilize the negative oxygens of the transition state, as a similar residue (Lysine) has been shown to carry out such function in RNase A. Alternatively, it could interact with the RNA backbone. The other polar residues, located in between the catalytic histidine and the conserved arginine, are thought to further augment the active site. (2,4) It is worth noting that, as in most CRISPR associated RNases, nuclease activity at this site is metal-dependent, as target cleavage only occurs in the presence of a certain concentration of Mg2+ and is abolished after addition of EDTA.(5) This feature contrasts with most HEPN nucleases, where activity is metal-independent. A CRISPR associated RNase, Csx2 from Pyrococcus furious, contains a Zn2+ within its HEPN domain, suggesting a possible role of a divalent cation in further stabilizing the reaction intermediate (2). | HEPNs (Higher Eukaryotes and Prokaryotes Nucleotide-binding domain) are alpha helical domains, many of which are present in RNA maturation systems and related biological conflicts and most of which contain a catalytic Rx4-6H active site. Cas 13b <scene name='81/817991/Hepn1/1'>HEPN1</scene> domain is composed of 12 linearly connected alpha helices flexible loop between them, whereas <scene name='81/817991/Hepn2/1'>HEPN2</scene> contains nine alpha helices, several short beta strands and beta-hairpin with positively charged residues at the tip. In accordance with the conserved active site from HEPNs, a Rx4-6H site is present, where active residues locate within both HEPN1 (<scene name='81/817991/Hepn1_active_site/1'>R156, N157, H161</scene>) and HEPN2 (<scene name='81/817991/Hepn2_active_site/1'>R1068, N1069, H1073</scene>) domains. One of the two histidines is thought to act as a base, inducing the ribose 2’OH to attack the phosphodiester linkage. The conserved arginine could stabilize the negative oxygens of the transition state, as a similar residue (Lysine) has been shown to carry out such function in RNase A. Alternatively, it could interact with the RNA backbone. The other polar residues, located in between the catalytic histidine and the conserved arginine, are thought to further augment the active site. (2,4) It is worth noting that, as in most CRISPR associated RNases, nuclease activity at this site is metal-dependent, as target cleavage only occurs in the presence of a certain concentration of Mg2+ and is abolished after addition of EDTA.(5) This feature contrasts with most HEPN nucleases, where activity is metal-independent. A CRISPR associated RNase, Csx2 from Pyrococcus furious, contains a Zn2+ within its HEPN domain, suggesting a possible role of a divalent cation in further stabilizing the reaction intermediate (2). | ||
| - | [[Image:Act1.png|550px|left|thumb| Fig. | + | [[Image:Act1.png|550px|left|thumb| Fig.2 HEPN nuclease site]] |
A highly conserved inter-domain linker (<scene name='81/817991/Idl/2'>IDL</scene>) connects HEPN1 with Helical-1 and spans a highly positivecentral <scene name='81/817991/Pbucas13b_central_channel/1'>inner channel</scene>, where crRNA lies isolated from the solvent. Helical-1 domain makes extensive contacts with the direct repeat of crRNA and minor interface contacts with both HEPNs and Lid domains, whereas Helical-2, which is composed of 11 helices, makes extensive contacts with HEPN1 and minor contacts with the extended beta-hairpin of the Lid domain. Between Helical1 and Helical2 domains there is a <scene name='81/817991/Channel_helicais/1'>side channel</scene> oppositely oriented from the Lid domain. This second side channel also is positively charged and establishes access to the unbound state crRNA from the solvent. | A highly conserved inter-domain linker (<scene name='81/817991/Idl/2'>IDL</scene>) connects HEPN1 with Helical-1 and spans a highly positivecentral <scene name='81/817991/Pbucas13b_central_channel/1'>inner channel</scene>, where crRNA lies isolated from the solvent. Helical-1 domain makes extensive contacts with the direct repeat of crRNA and minor interface contacts with both HEPNs and Lid domains, whereas Helical-2, which is composed of 11 helices, makes extensive contacts with HEPN1 and minor contacts with the extended beta-hairpin of the Lid domain. Between Helical1 and Helical2 domains there is a <scene name='81/817991/Channel_helicais/1'>side channel</scene> oppositely oriented from the Lid domain. This second side channel also is positively charged and establishes access to the unbound state crRNA from the solvent. | ||
| Line 26: | Line 26: | ||
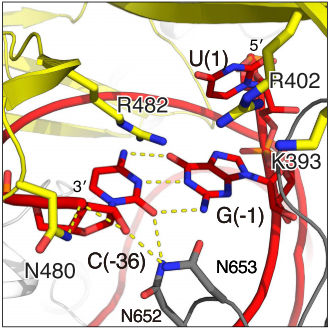
| - | [[Image:Act2.png|550px|right|thumb| Fig. | + | [[Image:Act2.png|550px|right|thumb| Fig. Second nuclease site]] |
| Line 38: | Line 38: | ||
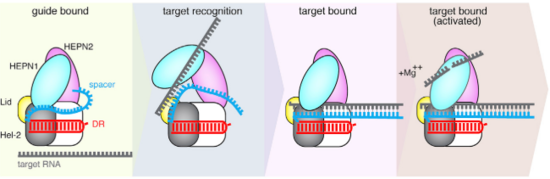
The Cas13b-crRNA complex is practically inaccessible for hybridization with target RNA unless a major conformational shift allows access to the central channel. An opening between the domains HEPN1 and Helical-2 has been suggested, since it would provide a sterically permissible route with charged amino acids that would direct the RNA to the Cas13b central cavity. Mutation in a residue on a Lid domain β-hairpin (D397) at the interface between HEPN1 and Helical2 decreases Cas13 knockdown activity, suggesting an additional role of this domain in directing the target RNA into the central channel. | The Cas13b-crRNA complex is practically inaccessible for hybridization with target RNA unless a major conformational shift allows access to the central channel. An opening between the domains HEPN1 and Helical-2 has been suggested, since it would provide a sterically permissible route with charged amino acids that would direct the RNA to the Cas13b central cavity. Mutation in a residue on a Lid domain β-hairpin (D397) at the interface between HEPN1 and Helical2 decreases Cas13 knockdown activity, suggesting an additional role of this domain in directing the target RNA into the central channel. | ||
| - | [[Image:Selection_039.png|550px|right|thumb| Fig. | + | [[Image:Selection_039.png|550px|right|thumb| Fig.4 Proposed mechanism of crRNA targeting by Cas13b]] |
The 3’end of target RNA is less tightly bound within the central channel and more tolerant of mismatches that the 5’end, thus being proposed as being initially recognized by the complex. In this suggested model the protein-crRNA complex initially probes the 3’RNA end of target RNA, allowing the opening of HEPN1 and Helical-2 domains and access to the central channel after complementarity is found. Then, the rest of the target RNA is hybridized. (Fig.2) | The 3’end of target RNA is less tightly bound within the central channel and more tolerant of mismatches that the 5’end, thus being proposed as being initially recognized by the complex. In this suggested model the protein-crRNA complex initially probes the 3’RNA end of target RNA, allowing the opening of HEPN1 and Helical-2 domains and access to the central channel after complementarity is found. Then, the rest of the target RNA is hybridized. (Fig.2) | ||
| Line 48: | Line 48: | ||
== Cas13b engineering == | == Cas13b engineering == | ||
| - | [[Image:ActLoop.png|700px|left|thumb| Fig. | + | [[Image:ActLoop.png|700px|left|thumb| Fig.5 HEPN2 conserved loop at active site]] |
The aforementioned preference for target RNA cleavage at dinucleotide sites is somewhat conserved among Cas 13 enzymes. A beta-hairpin loop located near the active site at HEPN2 domain is conserved among Cas13 from different species. Though the identities of its residues are variable, it is always located between highly conserved amino-acids (2) In PbuCas13b, mutants with altered residues at this loop that retained cleavage activity showed different cleavage preferences, and some of them even had increased preference for UU dinucleotides. SHERLOCK (Specific High-Sensitivity Enzymatic Reporter unLOCKing), an mRNA detection technique that takes advantage of the promiscuous cleavage activity of Cas13s is limited by the nucleobases identities of some of transcripts.(8) Therefore, Cas13b engineering at this conserved loop could potentially increase the repertoire of substrates for these type of techniques. | The aforementioned preference for target RNA cleavage at dinucleotide sites is somewhat conserved among Cas 13 enzymes. A beta-hairpin loop located near the active site at HEPN2 domain is conserved among Cas13 from different species. Though the identities of its residues are variable, it is always located between highly conserved amino-acids (2) In PbuCas13b, mutants with altered residues at this loop that retained cleavage activity showed different cleavage preferences, and some of them even had increased preference for UU dinucleotides. SHERLOCK (Specific High-Sensitivity Enzymatic Reporter unLOCKing), an mRNA detection technique that takes advantage of the promiscuous cleavage activity of Cas13s is limited by the nucleobases identities of some of transcripts.(8) Therefore, Cas13b engineering at this conserved loop could potentially increase the repertoire of substrates for these type of techniques. | ||
Revision as of 11:55, 17 June 2019
==Cas13b==
| |||||||||||
References