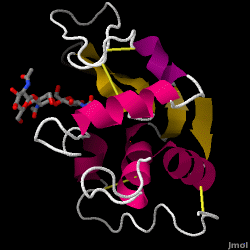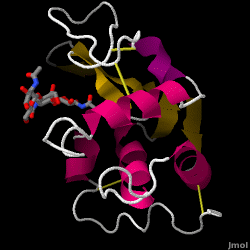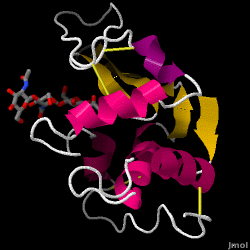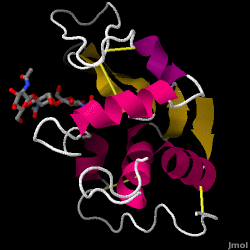
Hen Egg-White (HEW) Lysozyme
From Proteopedia
Revision as of 20:39, 20 June 2019
Lysozyme - also known as muramidase, or glycoside hydrolase - is a powerful enzyme of biological significance found in abundance in tears, saliva, and human milk. In humans, it is encoded in the LYZ gene. Although it is responsible for the initial digestion of starches in the mouth, it is most widely identified as a non-specific defense in gram positive bacteria and in many species of fungi. Due to its antibacterial effects, it is a strong component of the innate immune system, and is an important part of an infant's diet to ward off diarrheal diseases. Since it is a small, easily available, and highly stable protein containing only 129 amino acid residues, it has been subject to extensive research regarding its function and structure. Hen Egg White (HEW) Lysozyme is shown below.
Introduction
Lysozyme is an enzyme known for its unique ability to degrade the polysaccharide architecture of many kinds of cell walls, normally for the purpose of protection against bacterial infection[1]. Its effects were first noticed by Laschtschenko in 1909. It was officially characterized and termed “lysozyme” by Alexander Fleming, the same person credited for the accidental discovery of penicillin.
The characterization of lysozyme in 1922 by Alexander Fleming was providential in that the undertaken experiment related to the discovery of lysozyme was not geared toward any knowledge of such a protein as lysozyme [2]. During the unrelated experiment, nasal drippings were inadvertently introduced to a petri dish containing a bacterial culture, which culture consequently exhibited the results of an as yet unknown enzymatic reaction. The observation of this unknown reaction led to further research on the components of this reaction as well as to the corresponding identification of the newfound "lysozyme." Fleming's discovery was complemented by David C. Phillips' 1965 description of the three-dimensional structure of lysozyme via a 200 pm resolution model obtained from X-ray crystallography [3]. Phillips' work was especially groundbreaking since Phillips had managed to successfully elucidate the structure of an enzyme via X-ray crystallography - a feat that had never before been accomplished[4]. Phillips' research also led to the first sufficiently described enzymatic mechanism of catalytic action [5]. Thus, Phillips' elucidation of the function of lysozyme led Phillips to reach a more general conclusion on the diversity of enzymatic chemical action in relation to enzymatic structure. Clearly, the findings of Phillips as well as the more general historical development of the understanding of the structure and function of lysozyme have been paramount to the more general realm of enzyme chemistry.
See also
Function
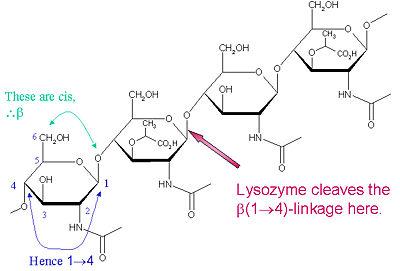
Lysozyme is known for damaging bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid (NAM) and N-acetyl-D-glucosamine (NAG) residues in peptidoglycan, and between N-acetyl-D-glucosamine residues in chitodextrins. In this way, lysozyme is efficient in lysing the cell walls of both bacteria and fungi. The location of cleavage for lysozyme on this architectural theme is the β(1-4) glycosidic linkage connecting the C1 carbon of NAM to the C4 carbon of NAG.
The particular substrate of preference for this cleavage type is a (NAG-NAM)₃ hexasaccharide, within which substrate occurs the cleaving target glycosidic bond, NAM₄-β-O-NAG₅. The individual hexasaccharide binding units are designated A-F, with NAM₄-β-O-NAG₅ glycosidic bond cleavage preference corresponding to a D-E unit glycosidic bond cleavage preference.
| |||||||||||
Mechanism
The lysozyme reaction is characterized by hydrolysis of the beta (1-4) glycosidic bond between NAM and NAG. Lysozyme has a very specific active site, which can bind only six sugar rings from a polysaccharide chain. Once lysozyme binds to this chain, it hydrolyzes them. These six sugar rings represent the ligand of lysozyme. The lysozyme then distorts the fourth sugar in the six-membered complex, producing stress on the molecule and breaking the glycosidic bond.
Lysozyme is best inhibited by small saccharides which act competitively with the natural substrate. The smaller saccharides will bind to the first three binding sites of the cleft (sites A-C), but will not reach sites D and E, where the enzyme cuts the glycosidic bond. So, the competitive inhibitor will stick in the cleft, not allowing the substrate to bind to the enzyme complex.[11] Several known inhibitors of lysozyme are: SDS, N-acetyl-D-glucosamine, and various alcohols and oxidizing agents.[12]
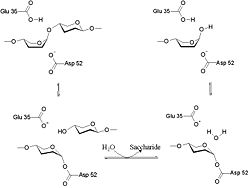
The lysozyme mechanism of action results in the hydrolysis of a glycoside (hence the familial distinction of lysozyme as a glycosylase[14]), which corresponds to the conversion of an acetal to a hemiacetal, which reaction (general degradation of glycosidic bond to units "capped" by newly formed hydroxyl groups) necessitates acid catalysis, since the conversion of acetal to hemiacetal involves the protonation of the reactant oxygen prior to actual bond cleavage. [15]. Furthermore, the transition state obtained from this protonation is a covalent, oxonium ion, intermediate that must obtain resonance stabilization. The need for some means of acid catalysis and covalent resonance stabilization is adequately provided by the Glu 35 and Asp 52 residues of lysozyme, respectively. The reaction mechanism of lysozyme is demonstrated on the left. The reaction begins at the upper left-hand side, and proceeds according to reaction arrows. Lysozyme works by hydrolyzing the glycosidic bond, distorting the bond between the NAM and NAG. This produces a glycosyl enzyme intermediate, which reacts with a water molecule to produce the product and the unchanged enzyme. The of lysozyme is formulated as a prominent cleft outlined by the two aforementioned catalytic amino acids, Glu 35 and Asp 52. The active site is geometrically bent to augment ligand binding, and the two amino acids interact with the ligand in the binding site. Asp52 is depicted in red, and Glu35 is depicted in green.
Applications of Lysozyme
Since lysozyme has been widely recognized for its antibacterial and antifungal properties, it has a wide variety of uses both in biochemical and pharmaceutical applications. In molecular biology, lysozyme is often used in the alkaline-lysis procedure for extracting and isolating plasmid DNA. It is used extensively in the pharmaceutical field for destroying gram-positive bacteria, and can be used to support already-existing immune defenses to fight bacterial infections. This enzyme is particularly important for preventing bacterial diseases in infants. Because of its antibacterial properties, lysozyme can also be used in the food industry to help prevent spoilage of foods.
See Also
References
- ↑ Lysozyme. 2010. Citizendium.org. http://en.citizendium.org/wiki/Lysozyme
- ↑ Lysozyme. 2008. Lysozyme.co.uk. http://lysozyme.co.uk/
- ↑ Lysozyme, 2008. Lysozyme.co.uk. http://lysozyme.co.uk/
- ↑ Bugg, T. 1997. An Introduction to Enzyme and Coenzyme Chemistry. Blackwell Science Ltd., Oxford
- ↑ 1967. Proc R Soc Lond B Bio 167 (1009): 389–401.
- ↑ Image from: http://www.vuw.ac.nz/staff/paul_teesdale-spittle/essentials/chapter-6/proteins/lysozyme.htm
- ↑ Johnson LN, Phillips DC. Structure of some crystalline lysozyme-inhibitor complexes determined by X-ray analysis at 6 Angstrom resolution. Nature. 1965 May 22;206(986):761-3. PMID:5840126
- ↑ Phillips, D. C. The hen egg white lysozyme molecule. Proc. Natl Acad. Sci. USA 57, 483-495 (1967)
- ↑ coordinates of the model kindly provided by Louise Johnson
- ↑ Vocadlo DJ, Davies GJ, Laine R, Withers SG. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature. 2001 Aug 23;412(6849):835-8. PMID:11518970 doi:10.1038/35090602
- ↑ http://mcdb-webarchive.mcdb.ucsb.edu/sears/biochemistry/tw-enz/lysozyme/HEWL/lysozyme-overview.htm
- ↑ http://www.worthington-biochem.com/ly/default.html
- ↑ Image from: http://www.google.com/imgres?imgurl=http://www.vuw.ac.nz/staff/paul_teesdale-spittle/essentials/chapter-6/pics-and-strucs/lysozyme-mech.gif&imgrefurl=http://www.vuw.ac.nz/staff/paul_teesdale-spittle/essentials/chapter-6/proteins/lysozyme.htm&usg=__ormapG4XKg-tR5GrMSOdSMTV4vE=&h=603&w=801&sz=7&hl=en&start=17&zoom=1&tbnid=nvr9gvFrUILDkM:&tbnh=143&tbnw=189&prev=/images%3Fq%3DThe%2Blysozyme%2Breaction%2Bmechanism%26um%3D1%26hl%3Den%26sa%3DN%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C304&um=1&itbs=1&iact=hc&vpx=521&vpy=349&dur=448&hovh=191&hovw=254&tx=140&ty=48&ei=JQ_LTPKzLIjCsAPkzt2KDg&oei=IA_LTP74OsG78gapm-GFAQ&esq=2&page=2&ndsp=18&ved=1t:429,r:2,s:17&biw=1280&bih=647
- ↑ Lysozyme, 2008. Lysozyme.co.uk. http://lysozyme.co.uk/
- ↑ Pratt, C.W., Voet, D., Voet, J.G. Fundamentals of Biochemistry - Life at the Molecular Level - Third Edition. Voet, Voet and Pratt, 2008.
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Michal Harel, Anne Goodling, Alexander Berchansky, Eric Martz, Joel L. Sussman, Karl Oberholser, Jaime Prilusky, John Ripollone, Daniel Kreider, Judy Voet, John S. de Banzie