GABA receptor
From Proteopedia
| Line 8: | Line 8: | ||
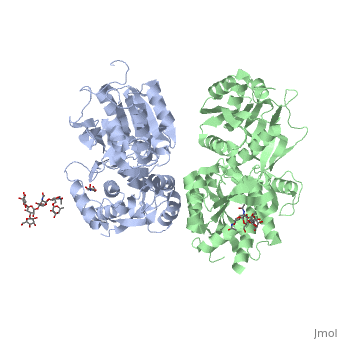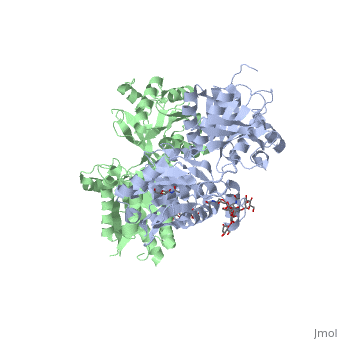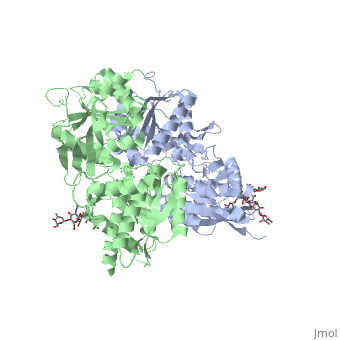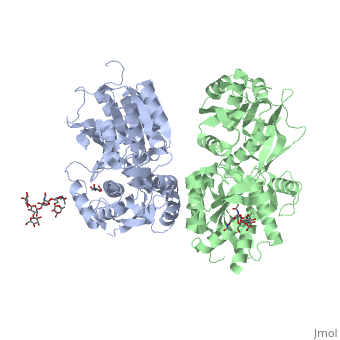
Ionotropic and metabotropic are the two major classes of GABA receptors abundant throughout neuronal cells (Cryan, 2005). Metabotropic GABAB receptors induce a change in membrane potential through the action of a second messenger pathway (Kerr, 1995). The GABAB receptor functions as a heterodimer of two subunits, GABAB1 (grey) and GABAB2 (green). Heterodimerization is accomplished through interactions of the coiled-coil motifs within the C-termini and interactions between the transmembrane and extracellular domains (Cryan, 2005). Additionally, there are two GABAB1 isoforms that differ at the N-termini where there are two sushi domains (Cryan, 2005). These sushi domains on the GABAB1 subunit are key to the receptor's interaction with other proteins as well as axonal signaling (Cryan, 2005). The two GABAB subunits link together as GABAB2 connects to GABAB1 via the <scene name='71/716457/Tyrosine1/1'>tyrosine residues</scene> on the intracellular C-termini to form the heterodimer GABAB receptor (Geng, 2013). These tyrosine residues are effective in linking the heterodimer as the base-stacking interactions and hydrogen bonding stabilize the quaternary structure (Geng, 2013). Lastly, the tyrosine residues, with adjacent <scene name='71/716457/Trp1/1'>lysine and tryptophan residues</scene>, are able to participate in hydrophobic interactions that contribute to the heterodimer's stability (Geng, 2013). | Ionotropic and metabotropic are the two major classes of GABA receptors abundant throughout neuronal cells (Cryan, 2005). Metabotropic GABAB receptors induce a change in membrane potential through the action of a second messenger pathway (Kerr, 1995). The GABAB receptor functions as a heterodimer of two subunits, GABAB1 (grey) and GABAB2 (green). Heterodimerization is accomplished through interactions of the coiled-coil motifs within the C-termini and interactions between the transmembrane and extracellular domains (Cryan, 2005). Additionally, there are two GABAB1 isoforms that differ at the N-termini where there are two sushi domains (Cryan, 2005). These sushi domains on the GABAB1 subunit are key to the receptor's interaction with other proteins as well as axonal signaling (Cryan, 2005). The two GABAB subunits link together as GABAB2 connects to GABAB1 via the <scene name='71/716457/Tyrosine1/1'>tyrosine residues</scene> on the intracellular C-termini to form the heterodimer GABAB receptor (Geng, 2013). These tyrosine residues are effective in linking the heterodimer as the base-stacking interactions and hydrogen bonding stabilize the quaternary structure (Geng, 2013). Lastly, the tyrosine residues, with adjacent <scene name='71/716457/Trp1/1'>lysine and tryptophan residues</scene>, are able to participate in hydrophobic interactions that contribute to the heterodimer's stability (Geng, 2013). | ||
| - | The GABAB receptor exists in the <scene name='71/716457/Gabab_rest/1'>resting state</scene> (PDB: | + | The GABAB receptor exists in the <scene name='71/716457/Gabab_rest/1'>resting state</scene> (PDB: [[4mqe]]) and the <scene name='71/716457/Active_state/1'>active state</scene> (PDB: [[4ms3]]) (Geng, 2013). Using the GABAB crystal structures, Geng et al. found that both subunits exist in open conformations while at rest. Upon binding with the agonist the GABAB1 subunit closes (Geng, 2013). Additionally, it was found that the agonist (i.e. GABA) is bound to the <scene name='71/716457/Active_site_iwith_gaba_bound/1'>active site</scene>, located at the interdomain crevice of the GABAB1 subunit due to an overlap of amino acid residues (Geng, 2013). This conformation change is noted by the visible reduction in space between GABAB subunits upon binding with GABA. |
== Function == | == Function == | ||
Revision as of 09:36, 17 December 2019
| |||||||||||
References
Bettler, B., Kaupmann, K., Mosbacher, J., & Gassmann, M. (2004). Molecular structure and physiological functions of GABAB receptors. Physiological reviews, 84(3), 835-867.
Citrome, L., Javitt, D., Kantrowitz, J. (2009). GABAB Receptors, Schizophrenia and Sleep Dysfunction. CNS Drugs, 23(8), 681-691.
Cryan, J.F., Kaupman, K. (2005). Don’t worry ‘B’ happy!: a role for GABAB receptors in anxiety and depression. Trends in Pharmacological Sciences, 26(1), 36-43.
Filip, M., Frankowska, M., et al., (2015). GABAB receptors as a therapuetic strategy in substance use disorders: Focus on positive allosteric modulators. Neuropharmacology. (38), 36-47.
Geng, Y., Bush, M., Mosyak, L., Wang, F., & Fan, Q. R. (2013). Structural mechanism of ligand activation in human GABAB receptor. Nature, 504(7479), 254-259.
Gumerov, V., Hegyi, H. (2015). MicroRNA-derived network analysis of differentially methylated genes in schizophrenia, implicating GABA receptor B1 [GABBR1] and protein kinase B [AKT1].Gumerov and Heygl Biology Direct. 10:59, 1-15.
Kerr, D. I. B., and J. Ong. "Clinical Potential of GABA B Receptor Modulators." CNS Drug Reviews. 11.3 (2005): 317-334.
Kerr, D. I. B., and J. Ong. "Gaba B receptors." Pharmacology & therapeutics. 67.2 (1995): 187-246.
Proteopedia Page Contributors and Editors (what is this?)
Jonathan Hurst, Gregory Holley, Alexander Berchansky, Michal Harel, Patrick Farrell, Joel L. Sussman, Karli Ribsam, Jaime Prilusky