Retinoblastoma-binding protein
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
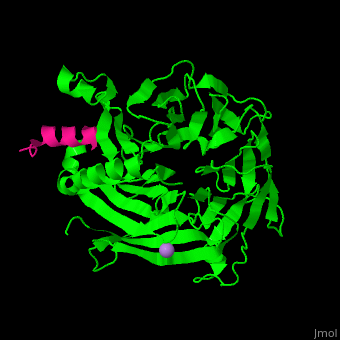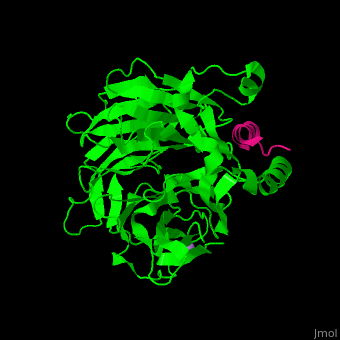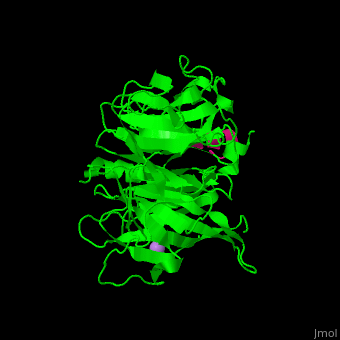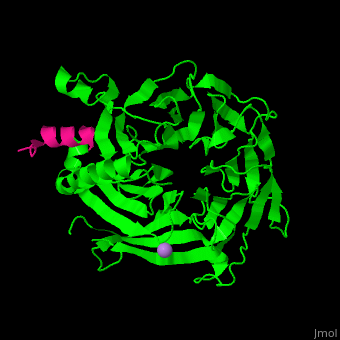
<StructureSection load='' size='350' side='right' scene='48/485630/Cv/1' caption='Human retinoblastoma-binding protein 7 (green) complex with histone H4 helix (magenta) and As ion (purple) [[3cfs]]'> | <StructureSection load='' size='350' side='right' scene='48/485630/Cv/1' caption='Human retinoblastoma-binding protein 7 (green) complex with histone H4 helix (magenta) and As ion (purple) [[3cfs]]'> | ||
| - | + | __TOC__ | |
== Function == | == Function == | ||
'''Retinoblastoma-binding protein''' (RBBP) or '''histone-binding protein''' are nuclear proteins implicated in the regulation of transcription and differentiation by the retinoblastoma tumor suppressor protein<ref>PMID:9697699</ref>.<br /> | '''Retinoblastoma-binding protein''' (RBBP) or '''histone-binding protein''' are nuclear proteins implicated in the regulation of transcription and differentiation by the retinoblastoma tumor suppressor protein<ref>PMID:9697699</ref>.<br /> | ||
| Line 6: | Line 6: | ||
* '''RBBP2''' demethylates histone H3 at lysine 4.<br /> | * '''RBBP2''' demethylates histone H3 at lysine 4.<br /> | ||
* '''RBBP2 homolog''' see KDM5B in [[Lysine-specific histone demethylase]].<br /> | * '''RBBP2 homolog''' see KDM5B in [[Lysine-specific histone demethylase]].<br /> | ||
| - | * '''RBBP5''' is involved in methylation and dimethylation of histone H3.<br /> | + | * '''RBBP5''' is involved in methylation and dimethylation of histone H3<ref>PMID:31544921</ref>.<br /> |
* '''RBBP6''' promotes p53 degradation<ref>PMID:21676486</ref>.<br /> | * '''RBBP6''' promotes p53 degradation<ref>PMID:21676486</ref>.<br /> | ||
* '''RBBP7''' is a WD-repeat protein that interacts with histone deacetylases<ref>PMID:12376095</ref>.<br /> | * '''RBBP7''' is a WD-repeat protein that interacts with histone deacetylases<ref>PMID:12376095</ref>.<br /> | ||
| Line 13: | Line 13: | ||
== Structural highlights == | == Structural highlights == | ||
<scene name='48/485630/Cv/6'>RBBP7 interacts with histone H4 in a pocket between its N-terminal α helix</scene> and its negatively charged <scene name='48/485630/Cv/7'>PP loop which contains 2 consecutive Pro residues</scene><ref>PMID:18571423</ref>.<br />. | <scene name='48/485630/Cv/6'>RBBP7 interacts with histone H4 in a pocket between its N-terminal α helix</scene> and its negatively charged <scene name='48/485630/Cv/7'>PP loop which contains 2 consecutive Pro residues</scene><ref>PMID:18571423</ref>.<br />. | ||
| + | ==3D structures of retinoblastoma-binding protein== | ||
| + | [[Retinoblastoma-binding protein 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
==3D structures of retinoblastoma-binding protein== | ==3D structures of retinoblastoma-binding protein== | ||
| Line 23: | Line 26: | ||
**[[2mam]] - hRBBP1 double tudor domain - NMR<br /> | **[[2mam]] - hRBBP1 double tudor domain - NMR<br /> | ||
| - | *Retinoblastoma-binding protein 2 | + | *Retinoblastoma-binding protein 2 or lysine-specific demethylase 5A or KDM5A; Domains – ARID 85-175; PHD 1609-1659; JMJ 1-87+348-588 |
**[[2jxj]] – hRBBP2 ARID domain + histone H3 – NMR<BR /> | **[[2jxj]] – hRBBP2 ARID domain + histone H3 – NMR<BR /> | ||
**[[2kgg]] – hRBBP2 C terminal PHD zinc finger – NMR<BR /> | **[[2kgg]] – hRBBP2 C terminal PHD zinc finger – NMR<BR /> | ||
**[[2kgi]] - hRBBP2 C terminal PHD zinc finger + histone H3 – NMR<BR /> | **[[2kgi]] - hRBBP2 C terminal PHD zinc finger + histone H3 – NMR<BR /> | ||
| - | **[[3gl6]] - hRBBP2 C terminal PHD zinc finger + histone H3 N terminal | + | **[[3gl6]] - hRBBP2 C terminal PHD zinc finger + histone H3 N terminal<br /> |
| + | **[[6bgu]], [[6bgv]] [[6bgw]], [[6bgx]], [[6bgy]], [[6bgz]] [[6bh0]], [[6bh1]], [[6bh2]], [[6bh3]] [[6bh4]], [[6bh5]], [[6dq4]], [[6dq5]], [[6dq6]], [[6dq7]], [[6dq8]], [[6dq9]], [[6dqa]], [[6dqb]], [[6dqc]], [[6dqd]], [[6dqe]], [[6dqf]], [[6ein]], [[6eiu]], [[6eiy]], [[6ej0]], [[6ej1]] – hRBBP2 JMJ domain + inhibitor<br /> | ||
*Retinoblastoma-binding protein 4 or histone-binding protein RBBP4 | *Retinoblastoma-binding protein 4 or histone-binding protein RBBP4 | ||
| Line 39: | Line 43: | ||
**[[6bw3]] – hRBBP4 + Mds1 peptide<br /> | **[[6bw3]] – hRBBP4 + Mds1 peptide<br /> | ||
**[[5y1u]] – hRBBP4 + Aebp2 peptide<br /> | **[[5y1u]] – hRBBP4 + Aebp2 peptide<br /> | ||
| + | **[[6bw4]] – hRBBP4 + Znf16 peptide<br /> | ||
**[[5xxq]] – hRBBP4 + Znf827 peptide<br /> | **[[5xxq]] – hRBBP4 + Znf827 peptide<br /> | ||
**[[5wak]] – hRBBP4 + Suz12 peptide<br /> | **[[5wak]] – hRBBP4 + Suz12 peptide<br /> | ||
| Line 45: | Line 50: | ||
**[[5fxy]], [[6g16]] – hRBBP4 + MTA1 protein <br /> | **[[5fxy]], [[6g16]] – hRBBP4 + MTA1 protein <br /> | ||
**[[5wai]] – hRBBP4 + Suz12 + Jumonji + Aebp2 <br /> | **[[5wai]] – hRBBP4 + Suz12 + Jumonji + Aebp2 <br /> | ||
| - | **[[6c23]] – hRBBP4 + Suz12 + Jumonji + Ezh2 + Eed + Jarid2 + Aebp2 – Cryo EM <br /> | + | **[[6c23]], [[6c24]] – hRBBP4 + Suz12 + Jumonji + Ezh2 + Eed + Jarid2 + Aebp2 – Cryo EM <br /> |
| + | |||
| + | *RBBP5 | ||
| + | |||
| + | **[[6km7]] – hRBBP5<br /> | ||
| + | **[[6kiu]], [[6kiv]], [[6kix]], [[6kiz]] – hRBBP5 in MLL1-UBNCP complex – Cryo EM<br /> | ||
| + | **[[6kiw]] – hRBBP5 in MLL3-UBNCP complex – Cryo EM<br /> | ||
*Retinoblastoma-binding protein 6 | *Retinoblastoma-binding protein 6 | ||
Revision as of 10:23, 10 December 2019
| |||||||||||
3D structures of retinoblastoma-binding protein
Updated on 10-December-2019
References
- ↑ Woitach JT, Zhang M, Niu CH, Thorgeirsson SS. A retinoblastoma-binding protein that affects cell-cycle control and confers transforming ability. Nat Genet. 1998 Aug;19(4):371-4. PMID:9697699 doi:http://dx.doi.org/10.1038/1258
- ↑ Fattaey AR, Helin K, Dembski MS, Dyson N, Harlow E, Vuocolo GA, Hanobik MG, Haskell KM, Oliff A, Defeo-Jones D, et al.. Characterization of the retinoblastoma binding proteins RBP1 and RBP2. Oncogene. 1993 Nov;8(11):3149-56. PMID:8414517
- ↑ Han J, Li T, Li Y, Li M, Wang X, Peng C, Su C, Li N, Li Y, Xu Y, Chen Y. The internal interaction in RBBP5 regulates assembly and activity of MLL1 methyltransferase complex. Nucleic Acids Res. 2019 Sep 23. pii: 5572575. doi: 10.1093/nar/gkz819. PMID:31544921 doi:http://dx.doi.org/10.1093/nar/gkz819
- ↑ Motadi LR, Bhoola KD, Dlamini Z. Expression and function of retinoblastoma binding protein 6 (RBBP6) in human lung cancer. Immunobiology. 2011 Oct;216(10):1065-73. doi: 10.1016/j.imbio.2011.05.004. Epub, 2011 May 17. PMID:21676486 doi:http://dx.doi.org/10.1016/j.imbio.2011.05.004
- ↑ Yang J, Kiefer S, Rauchman M. Characterization of the gene encoding mouse retinoblastoma binding protein-7, a component of chromatin-remodeling complexes. Genomics. 2002 Oct;80(4):407-15. PMID:12376095
- ↑ Vorobiev SM, Huang YJ, Seetharaman J, Xiao R, Acton TB, Montelione GT, Tong L. Human retinoblastoma binding protein 9, a serine hydrolase implicated in pancreatic cancers. Protein Pept Lett. 2012 Feb;19(2):194-7. PMID:21933118
- ↑ Murzina NV, Pei XY, Zhang W, Sparkes M, Vicente-Garcia J, Pratap JV, McLaughlin SH, Ben-Shahar TR, Verreault A, Luisi BF, Laue ED. Structural basis for the recognition of histone H4 by the histone-chaperone RbAp46. Structure. 2008 Jul;16(7):1077-85. Epub 2008 Jun 19. PMID:18571423 doi:http://dx.doi.org/10.1016/j.str.2008.05.006