We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Hemeproteins
From Proteopedia
(Difference between revisions)
| Line 46: | Line 46: | ||
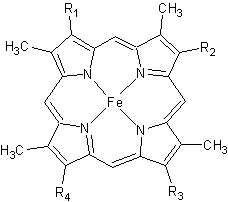
All members in the C-type cytochrome superfamily contain a heme prosthetic group that is covalently attached to the protein via two thioether bonds to cysteine residues. Most cytochromes ''c'' occur in a where the histidine residue is one of the two axial ligands of the heme iron.<ref name=main>PMID:18855424</ref><ref name=heme /> In monoheme cytochromes ''c'', the other axial position may be left vacant or be occupied by histidine or methionine residues; however, it can sometimes be occupied by cysteine or lysine residues.<ref name=main />. In ''Rm''cyt''c'', XX represents a threonine (Thr46) and an alanine residue (Ala47) that help form the loop 2 structure. | All members in the C-type cytochrome superfamily contain a heme prosthetic group that is covalently attached to the protein via two thioether bonds to cysteine residues. Most cytochromes ''c'' occur in a where the histidine residue is one of the two axial ligands of the heme iron.<ref name=main>PMID:18855424</ref><ref name=heme /> In monoheme cytochromes ''c'', the other axial position may be left vacant or be occupied by histidine or methionine residues; however, it can sometimes be occupied by cysteine or lysine residues.<ref name=main />. In ''Rm''cyt''c'', XX represents a threonine (Thr46) and an alanine residue (Ala47) that help form the loop 2 structure. | ||
| - | [[Image:heme.gif |frame|left| Figure 2. The tetrapyrrolic heme prosthetic group that can either be covalently attached to or closely associated with various proteins, such as cytochromes and other globin proteins. In ''Rm''cyt''c'', R2 is an ethyl group covalently attached to Cys 45, and R3 is a methyl group covalently attached to Cys48.]] | + | [[Image:heme.gif |frame|left|thumb|400px| Figure 2. The tetrapyrrolic heme prosthetic group that can either be covalently attached to or closely associated with various proteins, such as cytochromes and other globin proteins. In ''Rm''cyt''c'', R2 is an ethyl group covalently attached to Cys 45, and R3 is a methyl group covalently attached to Cys48.]] |
{{Clear}} | {{Clear}} | ||
The typical monoheme cyt ''c'' fold is formed by helices <scene name='Sandbox_Reserved_335/Helices/4'>A, C, and E</scene>. ''Rm''cyt''c'' contains seven α-helices that are folded around the heme, all connected by random coils.<ref name=main /> The heme group is axially coordinated by <scene name='Sandbox_Reserved_335/Axial/6'>His49 and Met100</scene>, and the disulfide linkages exist at <scene name='Sandbox_Reserved_335/Cys/1'>Cys45 and Cys48</scene>. The heme group in ''Rm''cyt''c'' is almost completely shielded from solvent due to it being in a mostly hydrophobic pocket. This pocket is formed in part by the seven helices surrounding the ring, but also by two structures that are uncommon in other cytochromes ''c''. First, a 21 amino acid extension of the N-terminal exists, forming <scene name='Sandbox_Reserved_335/Uncommon1/2'>α-helix A' and loop 1</scene>, which wraps around the back of the polypeptide.<ref name=main /> An extension resembling such has only been seen in ''Thermus thermophilus''; however, the extension occurs at the C-terminus rather than the N-terminus.<ref>doi:10.1006/jmbi.1997.1181</ref> A second rarity is that of <scene name='Sandbox_Reserved_335/Uncommon2/2'>helix B'</scene>, inserted between helix D and loop 3, that shields the bottom part of the heme from any solvent.<ref name=main /> In cytochrome ''c''<sub>2</sub> as well as mitochondrial cyt ''c'', a similar yet shorter helix was found, though this helix was present at a different place in the primary sequence. Also, instead of helix B', ''T. thermophilus'' contains a two-stranded [http://en.wikipedia.org/wiki/Beta_sheet β-sheet].<ref name=main /> One final note is the number of <scene name='Sandbox_Reserved_335/Met/1'>methionine</scene> residues that ''Rm''cyt''c'' contains. In general, cyt ''c'' contains about two methionines whereas ''Rm''cyt''c'' contains seven, located on the left of the heme.<ref name=main /> | The typical monoheme cyt ''c'' fold is formed by helices <scene name='Sandbox_Reserved_335/Helices/4'>A, C, and E</scene>. ''Rm''cyt''c'' contains seven α-helices that are folded around the heme, all connected by random coils.<ref name=main /> The heme group is axially coordinated by <scene name='Sandbox_Reserved_335/Axial/6'>His49 and Met100</scene>, and the disulfide linkages exist at <scene name='Sandbox_Reserved_335/Cys/1'>Cys45 and Cys48</scene>. The heme group in ''Rm''cyt''c'' is almost completely shielded from solvent due to it being in a mostly hydrophobic pocket. This pocket is formed in part by the seven helices surrounding the ring, but also by two structures that are uncommon in other cytochromes ''c''. First, a 21 amino acid extension of the N-terminal exists, forming <scene name='Sandbox_Reserved_335/Uncommon1/2'>α-helix A' and loop 1</scene>, which wraps around the back of the polypeptide.<ref name=main /> An extension resembling such has only been seen in ''Thermus thermophilus''; however, the extension occurs at the C-terminus rather than the N-terminus.<ref>doi:10.1006/jmbi.1997.1181</ref> A second rarity is that of <scene name='Sandbox_Reserved_335/Uncommon2/2'>helix B'</scene>, inserted between helix D and loop 3, that shields the bottom part of the heme from any solvent.<ref name=main /> In cytochrome ''c''<sub>2</sub> as well as mitochondrial cyt ''c'', a similar yet shorter helix was found, though this helix was present at a different place in the primary sequence. Also, instead of helix B', ''T. thermophilus'' contains a two-stranded [http://en.wikipedia.org/wiki/Beta_sheet β-sheet].<ref name=main /> One final note is the number of <scene name='Sandbox_Reserved_335/Met/1'>methionine</scene> residues that ''Rm''cyt''c'' contains. In general, cyt ''c'' contains about two methionines whereas ''Rm''cyt''c'' contains seven, located on the left of the heme.<ref name=main /> | ||
Revision as of 13:36, 3 November 2019
| |||||||||||
References
- ↑ Schenkman JB, Jansson I. The many roles of cytochrome b5. Pharmacol Ther. 2003 Feb;97(2):139-52. PMID:12559387
- ↑ Rodriguez-Maranon MJ, Qiu F, Stark RE, White SP, Zhang X, Foundling SI, Rodriguez V, Schilling CL 3rd, Bunce RA, Rivera M. 13C NMR spectroscopic and X-ray crystallographic study of the role played by mitochondrial cytochrome b5 heme propionates in the electrostatic binding to cytochrome c. Biochemistry. 1996 Dec 17;35(50):16378-90. PMID:8973214 doi:10.1021/bi961895o
- ↑ Crofts AR. The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol. 2004;66:689-733. PMID:14977419 doi:http://dx.doi.org/10.1146/annurev.physiol.66.032102.150251
- ↑ Berry EA, Huang LS, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-Ray Structure of Rhodobacter Capsulatus Cytochrome bc (1): Comparison with its Mitochondrial and Chloroplast Counterparts. Photosynth Res. 2004;81(3):251-75. PMID:16034531 doi:http://dx.doi.org/10.1023/B:PRES.0000036888.18223.0e
- ↑ Rajagopal BS, Wilson MT, Bendall DS, Howe CJ, Worrall JA. Structural and kinetic studies of imidazole binding to two members of the cytochrome c (6) family reveal an important role for a conserved heme pocket residue. J Biol Inorg Chem. 2011 Jan 26. PMID:21267610 doi:10.1007/s00775-011-0758-y
- ↑ Morelli X, Czjzek M, Hatchikian CE, Bornet O, Fontecilla-Camps JC, Palma NP, Moura JJ, Guerlesquin F. Structural model of the Fe-hydrogenase/cytochrome c553 complex combining transverse relaxation-optimized spectroscopy experiments and soft docking calculations. J Biol Chem. 2000 Jul 28;275(30):23204-10. PMID:10748163 doi:10.1074/jbc.M909835199
- ↑ Manole A, Kekilli D, Svistunenko DA, Wilson MT, Dobbin PS, Hough MA. Conformational control of the binding of diatomic gases to cytochrome c'. J Biol Inorg Chem. 2015 Mar 20. PMID:25792378 doi:http://dx.doi.org/10.1007/s00775-015-1253-7
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 Stelter M, Melo AM, Pereira MM, Gomes CM, Hreggvidsson GO, Hjorleifsdottir S, Saraiva LM, Teixeira M, Archer M. A Novel Type of Monoheme Cytochrome c: Biochemical and Structural Characterization at 1.23 A Resolution of Rhodothermus marinus Cytochrome c. Biochemistry. 2008 Oct 15. PMID:18855424 doi:10.1021/bi800999g
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedheme - ↑ Than ME, Hof P, Huber R, Bourenkov GP, Bartunik HD, Buse G, Soulimane T. Thermus thermophilus cytochrome-c552: A new highly thermostable cytochrome-c structure obtained by MAD phasing. J Mol Biol. 1997 Aug 29;271(4):629-44. PMID:9281430 doi:10.1006/jmbi.1997.1181
- ↑ Soares CM, Baptista AM, Pereira MM, Teixeira M. Investigation of protonatable residues in Rhodothermus marinus caa3 haem-copper oxygen reductase: comparison with Paracoccus denitrificans aa3 haem-copper oxygen reductase. J Biol Inorg Chem. 2004 Mar;9(2):124-34. Epub 2003 Dec 23. PMID:14691678 doi:10.1007/s00775-003-0509-9
- ↑ Pereira MM, Santana M, Teixeira M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim Biophys Acta. 2001 Jun 1;1505(2-3):185-208. PMID:11334784
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Karp, Gerald (2008). Cell and Molecular Biology (5th edition). Hoboken, NJ: John Wiley & Sons. ISBN 978-0470042175.