We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Protegrin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
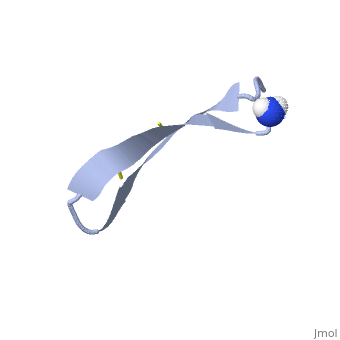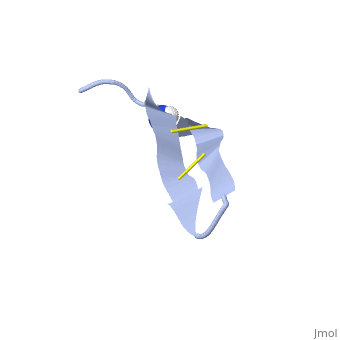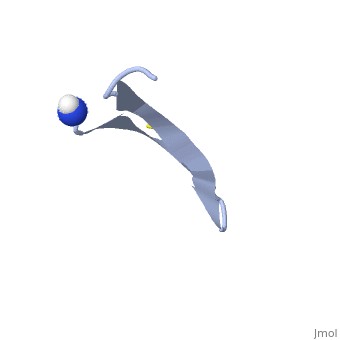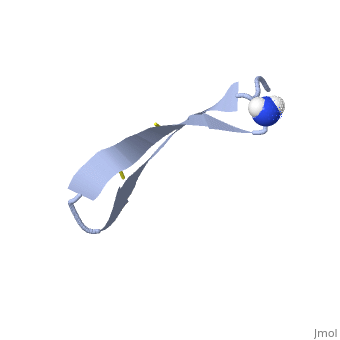
<StructureSection load='1pg1' size='350' side='right' caption='NMR structure of pig protegrin 1, [[1pg1]]' scene=''> | <StructureSection load='1pg1' size='350' side='right' caption='NMR structure of pig protegrin 1, [[1pg1]]' scene=''> | ||
| + | |||
| + | __Toc__ | ||
==About this Structure== | ==About this Structure== | ||
1PG1 is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Sus_scrofa Sus scrofa]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1PG1 OCA]. 1PG1 is an arginine and cysteine rich protein, which forms a double stranded anti-parallel β-sheet structure. The single chain forms a membrane-bound dimer, of structure [http://proteopedia.org/wiki/index.php/1zy6 1ZY6]. The structure of 1PG1 is similar to that of some other [http://en.wikipedia.org/wiki/Antimicrobial_peptide antimicrobial peptides] such as [http://en.wikipedia.org/wiki/Defensin defensins]<ref>Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity., Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME., J Clin Invest. 1989 Aug;84(2):553-61. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/2668334 2668334]</ref>. In one study comparing the susceptibility of [http://en.wikipedia.org/wiki/Chlamydia_trachomatis Chlamydia trachomatis] to 1PG1 and a similar defensin peptide, 1PG1 was shown to be significantly more effective at inactivating the bacteria<ref>Susceptibility of Chlamydia trachomatis to protegrins and defensins., Yasin B, Harwig SS, Lehrer RI, Wagar EA., Infect Immun. 1996 Mar;64(3):709-13. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/8641770 8641770]</ref>. The antimicrobial action of this protein is believed to be due to its ability to create pores in bacterial membranes causing ion leakage <ref>Membrane channel formation by antimicrobial protegrins., Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL, Biochim Biophys Acta. 1999 Aug 20;1420(1-2):23-9. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10446287 10446287]</ref>. This antimicrobial activity has given rise to the idea of using the peptide as a therapeutic for local or systemic infections<ref>Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity, D A Steinberg, M A Hurst, C A Fujii, A H Kung, J F Ho, F C Cheng, D J Loury, and J C Fiddes, Antimicrob Agents Chemother. 1997 August; 41(8): 1738–1742. PMCID: [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163996/ PMC163996]</ref>. The protegrin is synthesized as a ca. 149 amino acid precursor with a cathelin-like domain. | 1PG1 is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Sus_scrofa Sus scrofa]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1PG1 OCA]. 1PG1 is an arginine and cysteine rich protein, which forms a double stranded anti-parallel β-sheet structure. The single chain forms a membrane-bound dimer, of structure [http://proteopedia.org/wiki/index.php/1zy6 1ZY6]. The structure of 1PG1 is similar to that of some other [http://en.wikipedia.org/wiki/Antimicrobial_peptide antimicrobial peptides] such as [http://en.wikipedia.org/wiki/Defensin defensins]<ref>Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity., Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME., J Clin Invest. 1989 Aug;84(2):553-61. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/2668334 2668334]</ref>. In one study comparing the susceptibility of [http://en.wikipedia.org/wiki/Chlamydia_trachomatis Chlamydia trachomatis] to 1PG1 and a similar defensin peptide, 1PG1 was shown to be significantly more effective at inactivating the bacteria<ref>Susceptibility of Chlamydia trachomatis to protegrins and defensins., Yasin B, Harwig SS, Lehrer RI, Wagar EA., Infect Immun. 1996 Mar;64(3):709-13. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/8641770 8641770]</ref>. The antimicrobial action of this protein is believed to be due to its ability to create pores in bacterial membranes causing ion leakage <ref>Membrane channel formation by antimicrobial protegrins., Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL, Biochim Biophys Acta. 1999 Aug 20;1420(1-2):23-9. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10446287 10446287]</ref>. This antimicrobial activity has given rise to the idea of using the peptide as a therapeutic for local or systemic infections<ref>Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity, D A Steinberg, M A Hurst, C A Fujii, A H Kung, J F Ho, F C Cheng, D J Loury, and J C Fiddes, Antimicrob Agents Chemother. 1997 August; 41(8): 1738–1742. PMCID: [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163996/ PMC163996]</ref>. The protegrin is synthesized as a ca. 149 amino acid precursor with a cathelin-like domain. | ||
| Line 33: | Line 35: | ||
[[1pg1]], [[1zy6]] – pPRO1 – pig – NMR<BR /> | [[1pg1]], [[1zy6]] – pPRO1 – pig – NMR<BR /> | ||
| + | [[2muh]] - pPRO2 residues 131-146 - NMR<br /> | ||
[[1n5h]], [[1n5p]] – pPRO3 cathelin-like domain – NMR<br /> | [[1n5h]], [[1n5p]] – pPRO3 cathelin-like domain – NMR<br /> | ||
[[1kwi]], [[1lxe]], [[1pfp]] – pPRO3 cathelin-like domain <br /> | [[1kwi]], [[1lxe]], [[1pfp]] – pPRO3 cathelin-like domain <br /> | ||
Revision as of 06:53, 8 October 2020
| |||||||||||
References
Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes., Fahrner RL, Dieckmann T, Harwig SS, Lehrer RI, Eisenberg D, Feigon J, Chem Biol. 1996 Jul;3(7):543-50. PMID:8807886
3D structures of protegrin
Updated on 08-October-2020
1pg1, 1zy6 – pPRO1 – pig – NMR
2muh - pPRO2 residues 131-146 - NMR
1n5h, 1n5p – pPRO3 cathelin-like domain – NMR
1kwi, 1lxe, 1pfp – pPRO3 cathelin-like domain
2m2z - pPRO3 residues 131-148 - NMR
6qkf – pPtn-4 - NMR
- ↑ Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity., Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME., J Clin Invest. 1989 Aug;84(2):553-61. PMID: 2668334
- ↑ Susceptibility of Chlamydia trachomatis to protegrins and defensins., Yasin B, Harwig SS, Lehrer RI, Wagar EA., Infect Immun. 1996 Mar;64(3):709-13. PMID: 8641770
- ↑ Membrane channel formation by antimicrobial protegrins., Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL, Biochim Biophys Acta. 1999 Aug 20;1420(1-2):23-9. PMID:10446287
- ↑ Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity, D A Steinberg, M A Hurst, C A Fujii, A H Kung, J F Ho, F C Cheng, D J Loury, and J C Fiddes, Antimicrob Agents Chemother. 1997 August; 41(8): 1738–1742. PMCID: PMC163996
- ↑ NPG1. EBI QuickGO. http://www.ebi.ac.uk/QuickGO/GProtein?ac=P32194
- ↑ Protein: Protegrin 1 (PG1) from Pig (Sus scrofa). Structural Classification of Proteins. http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.ba.e.b.f.b.html
Created with the participation of Lee Tien.