Sandbox Reserved 1559
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
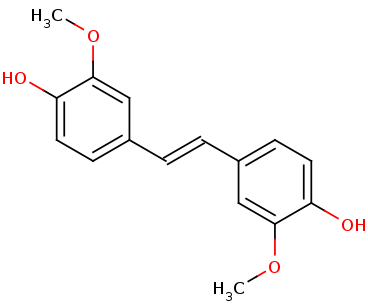
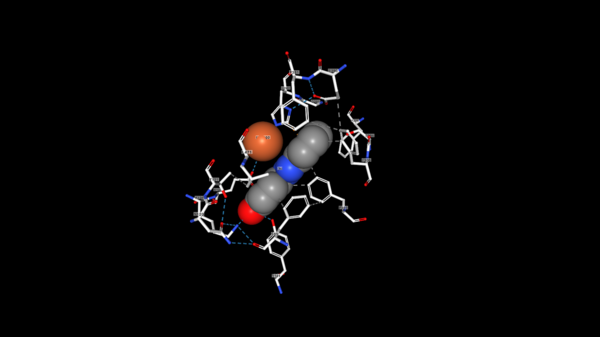
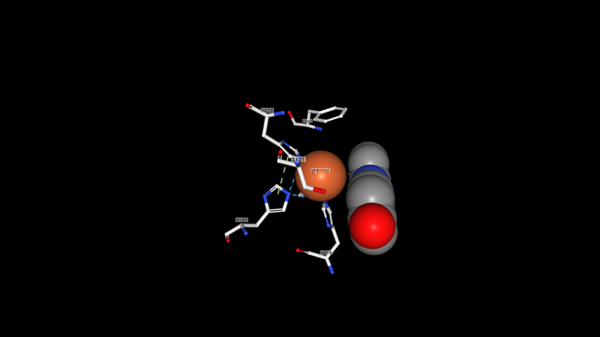
The tertiary structure also creates a metal binding site of Histidines to keep the iron molecule in place. There have been two mechanisms proposed for Lsd's. In one mechanism, the hydroxystillbenoid is activated via the enzyme-catalyzed deprotonation of the 4-hydroxy group, which then allows electron delocalization toward an Fe3+. In the other mechanism, π electron density from the double bond is redistributed to the iron-oxy complex to form an Fe2+ cation intermediate. Deprotonation of the hydroxyl is demanding for both mechanisms and is assisted by Lys134 and Tyr101. | The tertiary structure also creates a metal binding site of Histidines to keep the iron molecule in place. There have been two mechanisms proposed for Lsd's. In one mechanism, the hydroxystillbenoid is activated via the enzyme-catalyzed deprotonation of the 4-hydroxy group, which then allows electron delocalization toward an Fe3+. In the other mechanism, π electron density from the double bond is redistributed to the iron-oxy complex to form an Fe2+ cation intermediate. Deprotonation of the hydroxyl is demanding for both mechanisms and is assisted by Lys134 and Tyr101. | ||
| + | [[Image:Fe_View.png|600 px]] | ||
Revision as of 21:34, 29 November 2019
| This Sandbox is Reserved from Aug 26 through Dec 12, 2019 for use in the course CHEM 351 Biochemistry taught by Bonnie_Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1556 through Sandbox Reserved 1575. |
To get started:
More help: Help:Editing |
Overview
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644