Molecular Playground/Nickel Superoxide Dismutase
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
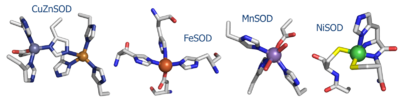
NiSOD shares no sequence homology with the [http://en.wikipedia.org/wiki/Superoxide_dismutase other known SOD's] ('''Fig. 1''').<sup><ref>PMID: 1094908</ref>-<ref>PMID: 11912934</ref></sup> Copper, iron, and manganese SOD are redox active in aqueous media at biological pH. Nickel is not, and requires the coordination of the two cysteine ligands to tune its redox potential, which is estimated to lie above 2 eV, far outside the biologically relevant redox potential necessary to oxidize or reduce superoxide [-160 to +879 eV].<ref>Uedsemma, M., Tamm, T. Density-functional theory calculations of aqueous redox potentials of fourth-period transition metals. J. Phys. Chem. A. 2003 Aug 4;107:9997-10003. Epub 2003 Oct 23. 10.1021/jp0362741</ref> The ligands employed in the redox-active metal center are distinct. Cu/Zn, Fe, and MnSOD employ only aspartic acids, waters, and histidines.<ref>PMID: 9878406</ref>, <ref>PMID: 1394426</ref>, <ref>PMID: 7849024</ref> In NiSOD, the nickel center is coordinated by the side chains of cysteine 2 and cysteine 6, as well as the N-terminal amine, the amide group of cysteine 2 and an axial histidine ligand ('''Fig. 1'''). <br /> | NiSOD shares no sequence homology with the [http://en.wikipedia.org/wiki/Superoxide_dismutase other known SOD's] ('''Fig. 1''').<sup><ref>PMID: 1094908</ref>-<ref>PMID: 11912934</ref></sup> Copper, iron, and manganese SOD are redox active in aqueous media at biological pH. Nickel is not, and requires the coordination of the two cysteine ligands to tune its redox potential, which is estimated to lie above 2 eV, far outside the biologically relevant redox potential necessary to oxidize or reduce superoxide [-160 to +879 eV].<ref>Uedsemma, M., Tamm, T. Density-functional theory calculations of aqueous redox potentials of fourth-period transition metals. J. Phys. Chem. A. 2003 Aug 4;107:9997-10003. Epub 2003 Oct 23. 10.1021/jp0362741</ref> The ligands employed in the redox-active metal center are distinct. Cu/Zn, Fe, and MnSOD employ only aspartic acids, waters, and histidines.<ref>PMID: 9878406</ref>, <ref>PMID: 1394426</ref>, <ref>PMID: 7849024</ref> In NiSOD, the nickel center is coordinated by the side chains of cysteine 2 and cysteine 6, as well as the N-terminal amine, the amide group of cysteine 2 and an axial histidine ligand ('''Fig. 1'''). <br /> | ||
| - | [[Image:SODs.png| | + | [[Image:SODs.png|400 px|thumb|'''Fig. 1:''' Active site structures of the four known SOD's.]] <br /> |
===NiSOD Structure=== | ===NiSOD Structure=== | ||
Revision as of 10:12, 29 December 2019
| |||||||||||
References
- ↑ Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147-59. PMID:1094908 doi:http://dx.doi.org/10.1146/annurev.bi.44.070175.001051
- ↑ Lee JW, Roe JH, Kang SO. Nickel-containing superoxide dismutase. Methods Enzymol. 2002;349:90-101. PMID:11912934
- ↑ Uedsemma, M., Tamm, T. Density-functional theory calculations of aqueous redox potentials of fourth-period transition metals. J. Phys. Chem. A. 2003 Aug 4;107:9997-10003. Epub 2003 Oct 23. 10.1021/jp0362741
- ↑ Bordo D, Matak D, Djinovic-Carugo K, Rosano C, Pesce A, Bolognesi M, Stroppolo ME, Falconi M, Battistoni A, Desideri A. Evolutionary constraints for dimer formation in prokaryotic Cu,Zn superoxide dismutase. J Mol Biol. 1999 Jan 8;285(1):283-96. PMID:9878406 doi:10.1006/jmbi.1998.2267
- ↑ Borgstahl GE, Parge HE, Hickey MJ, Beyer WF Jr, Hallewell RA, Tainer JA. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992 Oct 2;71(1):107-18. PMID:1394426
- ↑ Lah MS, Dixon MM, Pattridge KA, Stallings WC, Fee JA, Ludwig ML. Structure-function in Escherichia coli iron superoxide dismutase: comparisons with the manganese enzyme from Thermus thermophilus. Biochemistry. 1995 Feb 7;34(5):1646-60. PMID:7849024