This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1102
From Proteopedia
| Line 7: | Line 7: | ||
== Function == | == Function == | ||
| - | Zika is an [https://en.wikipedia.org/wiki/Double-stranded_RNA_viruses double-stranded RNA virus]. The genome replication of double-Stranded RNA virus need intervention of [https://en.wikipedia.org/wiki/Helicase helicase]. | + | Zika is an [https://en.wikipedia.org/wiki/Double-stranded_RNA_viruses double-stranded RNA virus]. The [https://en.wikipedia.org/wiki/Viral_replication genome replication] of double-Stranded RNA virus need intervention of [https://en.wikipedia.org/wiki/Helicase helicase]. |
| - | Protein from Zika | + | Protein from Zika consists of a protease domain at its [https://en.wikipedia.org/wiki/N-terminus N terminus] and a helicase domain at its [https://en.wikipedia.org/wiki/C-terminus C terminus] (8–10). The multifunctional helicase, belonging to the superfamily 2 (SF2) helicase family, is an essential enzyme involved in the cycles of ATP hydrolysis and behaves as a motor protein in RNA unwinding (11, 12). |
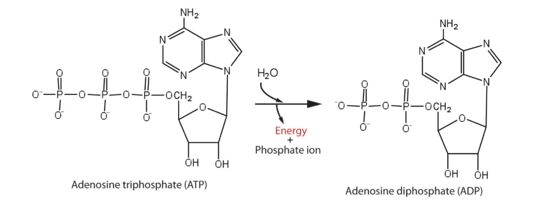
helicase domain that has 5'-triphosphatase activity of Zika performs the critical and indispensable function of unwinding double-stranded RNA during Zika genome replication. Helicase performs ATP hydrolysis at the 5′ end of RNA to generate energy. The phosphorus atom of the γ-phosphate group of the ATP molecule is attacked by water molecule; then, the γ-phosphate group is released with ADP. With the use of the energy derived from ATP hydrolysis, helicase translocates along nucleic acid strands, unwinds the double-stranded RNA genome. | helicase domain that has 5'-triphosphatase activity of Zika performs the critical and indispensable function of unwinding double-stranded RNA during Zika genome replication. Helicase performs ATP hydrolysis at the 5′ end of RNA to generate energy. The phosphorus atom of the γ-phosphate group of the ATP molecule is attacked by water molecule; then, the γ-phosphate group is released with ADP. With the use of the energy derived from ATP hydrolysis, helicase translocates along nucleic acid strands, unwinds the double-stranded RNA genome. | ||
Revision as of 15:43, 12 January 2020
| This Sandbox is Reserved from 25/11/2019, through 30/9/2020 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1091 through Sandbox Reserved 1115. |
To get started:
More help: Help:Editing |
Contents |
5y6n- Zika virus helicase in complex with ADP
5y6n is a 1 chain protein structure. It’s the only helicase belongs to zika virus.
Function
Zika is an double-stranded RNA virus. The genome replication of double-Stranded RNA virus need intervention of helicase.
Protein from Zika consists of a protease domain at its N terminus and a helicase domain at its C terminus (8–10). The multifunctional helicase, belonging to the superfamily 2 (SF2) helicase family, is an essential enzyme involved in the cycles of ATP hydrolysis and behaves as a motor protein in RNA unwinding (11, 12).
helicase domain that has 5'-triphosphatase activity of Zika performs the critical and indispensable function of unwinding double-stranded RNA during Zika genome replication. Helicase performs ATP hydrolysis at the 5′ end of RNA to generate energy. The phosphorus atom of the γ-phosphate group of the ATP molecule is attacked by water molecule; then, the γ-phosphate group is released with ADP. With the use of the energy derived from ATP hydrolysis, helicase translocates along nucleic acid strands, unwinds the double-stranded RNA genome.
Which is an essential step for viral RNA replication (14–16). It then provides conditions for the polymerization of RNA by an RNA-dependent RNA polymerase and the methylation of RNA by methyltransferase, which is crucial for Zika replication (13). In addition, it has been reported that the ATPase activity could alter viral replicative capacity and efficiency and consequently change the host innate immune response (17, 18).
Of these processes, ATP hydrolysis represents the most basic event; however, its dynamic mechanisms remain largely unknown, impeding the further understanding of the function of Zika helicase.
Disease
Relevance
Structural highlights
5y6n is a 1 chain protein of 438 residues. There are 2 main activities: ATP binding and helicase activity Helicase from Zika virus with a specific domain : a serine protease. It is a specific class of protein that hydrolysis peptide bonds from proteins.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>