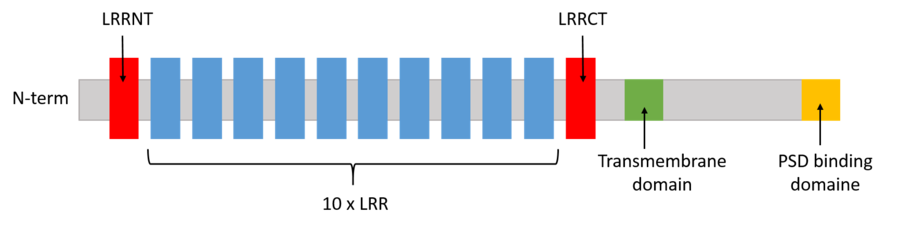Sandbox Reserved 1093
From Proteopedia
| Line 1: | Line 1: | ||
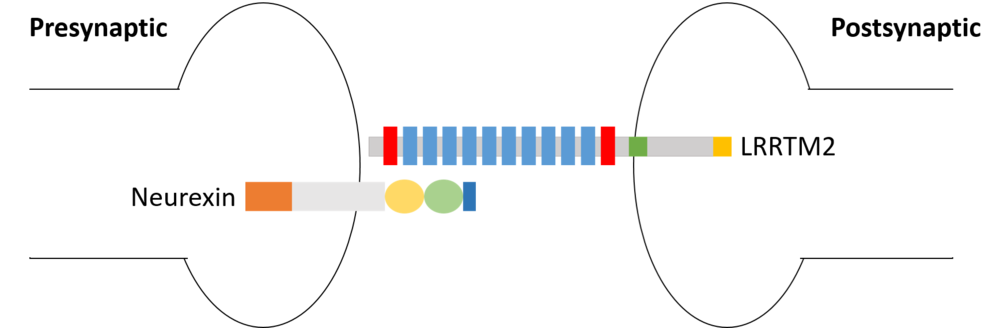
| - | <scene name='82/829346/Lrrtm2/3'>LRRTM2</scene> is a transmembrane protein that can be found in human neurons. It functions as postsynaptic organizers that induce excitatory synapses. LRRTM2 is prominently expressed in deep layers, rather than superficial layers, of the cerebral cortex. LRRTM2 specifically localizes in excitatory synapses, and not in inhibitory synapses. In addition, LRRTMs interact with neurexins[http://proteopedia.org/wiki/index.php/Neurexin]to bridge the synaptic cleft. | + | <scene name='82/829346/Lrrtm2/3'>LRRTM2</scene> is a transmembrane protein that can be found in human neurons. It functions as postsynaptic organizers that induce excitatory synapses. LRRTM2 is prominently expressed in deep layers (hippocampal neurons mostly), rather than superficial layers, of the cerebral cortex. LRRTM2 specifically localizes in excitatory synapses, and not in inhibitory synapses. In addition, LRRTMs interact with neurexins[http://proteopedia.org/wiki/index.php/Neurexin]to bridge the synaptic cleft. |
[[Image:LRRTM2+neurexin.png|1000px|left]] | [[Image:LRRTM2+neurexin.png|1000px|left]] | ||
| Line 27: | Line 27: | ||
== Function == | == Function == | ||
| + | |||
| + | LRRTM2 protein play a key role in the regulation of the synaptic fonctions and development by interracting with various proteins both inside and outside the neuron cell. | ||
1) Interaction with intracellular proteins | 1) Interaction with intracellular proteins | ||
| - | + | It has been shown that the binding of LRRTM2 with PSD-95 on the C-term fixation site was responsible for the regulation of AMPA receptor expression. The AMPA receptors ( AMPARs) are transmembrane receptors located on the postsynaptic membrane that interact with glutamates (neurotransmittters). So, the expression of LRRTM2 in the neurons of the hippocampus have a direct link with the density of excitatory synapses. The greater the quantity of LRRTM2, the greater the density of excitatory synapses. Thus, LRRTM2 has a direct influence on the glutamatergic synaptic transmission strength. | |
| - | It has been shown that the binding | + | It has also been noted that the repression of LRRTM2 induced a decrease in the density of PSD-95. Thus, LRRTM2 recruits PSD-95 at postsynaptic density and then binds to PSD-95 via the ECFV cytoplasmic motif. The interaction of PSD-95 with glutamate receptors, located at the postsynaptic membrane, as well as with other synaptic synaptic proteins. |
| - | It has also been noted that the repression of LRRTM2 induced a decrease in the density of PSD-95. Thus, LRRTM2 recruits PSD-95 at postsynaptic density and then binds to PSD-95 via the ECFV cytoplasmic motif. The interaction of PSD-95 with glutamate receptors, located at the postsynaptic | + | By the interaction with important postsynaptic components, LRRTM2 turns out to be crucial in the regulation of the postsynaptic fonctions and plasticity. |
| - | + | ||
| - | By the interaction with important postsynaptic | + | |
2) Interaction with extracellular proteins | 2) Interaction with extracellular proteins | ||
| - | + | On the N-term fixation site, LRRTM2 binds specifically to Neurexin1 α and β. The affinity of the binding depends on the splicing of the insert SS4 of both neurexins. This binding plays a critical role in the formation of excitatory synapses as it briges the synaptic cleft. Without Neurexin1, LRRTM2 can't act on presynaptic differentiation leading to a reduction in the amount of excitatory synapses. In addition, the presynaptic receptors Neurexin 1 α and β are known to be receptors for Neurologine 1, a protein similar to LRRTM2. Neurologine 1 also regulates the formation of excitatory synapses. | |
Revision as of 17:50, 16 January 2020
is a transmembrane protein that can be found in human neurons. It functions as postsynaptic organizers that induce excitatory synapses. LRRTM2 is prominently expressed in deep layers (hippocampal neurons mostly), rather than superficial layers, of the cerebral cortex. LRRTM2 specifically localizes in excitatory synapses, and not in inhibitory synapses. In addition, LRRTMs interact with neurexins[1]to bridge the synaptic cleft.
Contents |
Structural highlights
| |||||||||||
Function
LRRTM2 protein play a key role in the regulation of the synaptic fonctions and development by interracting with various proteins both inside and outside the neuron cell.
1) Interaction with intracellular proteins
It has been shown that the binding of LRRTM2 with PSD-95 on the C-term fixation site was responsible for the regulation of AMPA receptor expression. The AMPA receptors ( AMPARs) are transmembrane receptors located on the postsynaptic membrane that interact with glutamates (neurotransmittters). So, the expression of LRRTM2 in the neurons of the hippocampus have a direct link with the density of excitatory synapses. The greater the quantity of LRRTM2, the greater the density of excitatory synapses. Thus, LRRTM2 has a direct influence on the glutamatergic synaptic transmission strength. It has also been noted that the repression of LRRTM2 induced a decrease in the density of PSD-95. Thus, LRRTM2 recruits PSD-95 at postsynaptic density and then binds to PSD-95 via the ECFV cytoplasmic motif. The interaction of PSD-95 with glutamate receptors, located at the postsynaptic membrane, as well as with other synaptic synaptic proteins. By the interaction with important postsynaptic components, LRRTM2 turns out to be crucial in the regulation of the postsynaptic fonctions and plasticity.
2) Interaction with extracellular proteins
On the N-term fixation site, LRRTM2 binds specifically to Neurexin1 α and β. The affinity of the binding depends on the splicing of the insert SS4 of both neurexins. This binding plays a critical role in the formation of excitatory synapses as it briges the synaptic cleft. Without Neurexin1, LRRTM2 can't act on presynaptic differentiation leading to a reduction in the amount of excitatory synapses. In addition, the presynaptic receptors Neurexin 1 α and β are known to be receptors for Neurologine 1, a protein similar to LRRTM2. Neurologine 1 also regulates the formation of excitatory synapses.
Related Structures
LRRTMs Family
All four members of the human LRRTM family are highly similar in their LRR domains with >55% sequence identity. But only LRRTM1 and LRRTM2 have been extensively studied in the context of the interaction with Nrxn. This is due to the fact that some critical residues for binding with Nrxn1β [2] have been replaced in LRRTM3 and LRRTM4, such as , , and . The replacement of Glu348 by Val in LRRTM3 is likely to prevent of the interaction between Ca2+ and Nrxn1β. It is possible that other specific residue(s) of LRRTM3/4 may also prevent the binding.
Ligands
The structure of the complex [3]is being determined by co-crystallisation. A mutation from His 355 to Ala 355 without affecting the complex structure is necessary to maintain the stability of the crystal.
Other synaptic organisers
Neuroligins (NLs)
LRRTM2 bind to Neurexins 1, 2 and 3 ︎and ︎a variant region at splice site 4 in the LNS. As the variant region lacking a 30 amino acid insert (-S4), LRRTM2 cannot induce presynaptic differentiation in neurons. On the contrary, Neuroligin1 binds to Neurexins 1, 2, and 3, has a higher affinity with Neurexin 1 (-S4) than with Neurexin 1 (+S4)
GluD2 [[4]]
Neurexins
Neurexins (Nrxns) [5]is a family of the presynaptic organizer which interact with several postsynaptic organizers such as LRRTM2. There are three Neurexin genes in vertebrates, each corresponding to a different promoter type. Neurexins are characterized by their laminin-neurexin-sex hormone (LNS) domains. ︎ have six whereas ︎ have a single LNS domain. The α-helical conformation causes severe steric hindrance with the bound LRRTM2, whereas the β-stranded conformation causes no obvious steric hindrance.
Disease
A large number of researches shows that LRRTM2 is related to bipolar disorder. A deletion (240 kb) at 5q31 chromosomal region containing LRRTM2 and CTNNA1 has been shown to be related to intellectual disability and developmental delay.
References
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6160412/ https://pdf.sciencedirectassets.com/271175/1-s2.0-S0168010217X00047/1-s2.0-S0168010216302176/main.pdf?X-Amz-Security-Token=IQoJb3JpZ2luX2VjEIn%2F%2F%2F%2F%2F%2F%2F%2F%2F%2FwEaCXVzLWVhc3QtMSJIMEYCIQD9y3Gq4IpA3WUL5u%2Fkg2WA1Xkc%2FecGOKwBvrh87jA43QIhALaUG9EO6UsgdfDX4BdAFQdHgdcRASV4GY5gcp4MpAU1KrQDCBEQAhoMMDU5MDAzNTQ2ODY1IgxX8k4XAFbLEwxUIxEqkQMGYQSzdSg4KJygQuQhcirZ5z1dcUiJllkhebembjnSpLm2HgwQyXo8kS7OyOG4LrZK%2FpuVLgcwKJPzhlzfC8hvL4XkbdOHINPOAHjqrQAZfDUTyerG37EygqlyBH3ozWLj6bBRzb4qjtTKHiJXIVViFUwE4kLnUx%2BG1P9nlMZKiKwjTTZANO6qdo02b0eBH5wtGZXkTThixMrkac5AkC%2F6lv55c6GQkaGJ7QFUTzuMDhw1jnjgjh3SYEvL3zSXYMMmK9cdAvX47pXUxrx2upPm%2B1b6tXK9t%2BtcMhmGekMeq%2BQ4vgAGco9W47wKMZckdEtWsBwLD0ouczegSiUsY2j7%2Bkbrq3doyu8IVfj2trxYgsDhsot0o9LFT6vU4OcBDau43lRiWt28NL9taG2HVIr6S0JpdQrG5GnhP%2B9JBw0NzpNienHWZklAjtH5Yf2UdelMJoVxQVhA5Wt%2BxJdRYEa96OmlsXN%2FPrTFepkkdCM8oRnTDPYIsrzdNE7ztyE%2BKdycj4X5AmvBRpmLhlhj8JCajzDXhObwBTrqAbLxyZNQ%2F%2BztbwIwb1i%2FYTwtf4elBbvP75%2F%2BPavRxseS9SYjHMist7P2A3ic9sXaaRIVHQ%2BiNot7dRJXTHEmnQm%2Fpv7wJS%2BL3FrBNKhq2dtEs3wZwYiSkGRlQcFqq9B%2F6z%2FEpYEhZyY%2BXvVFlPy3UBFWSae%2B7sI6WH7Ioqesc5tp20wMy%2B86dD5OmNOpvKjmiRLEEzQU3yDhDHJbx3MEUlMTaugj2oDUpQwOhwwHGH5RWTUcyZQY2%2FZXTfvrmb4CbE9z%2BStJMmKQNGFFSfwmHo5tGI5vdQhOhljI0Jbg6Uarx7PMrUlHTCMy%2FA%3D%3D&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Date=20200111T090940Z&X-Amz-SignedHeaders=host&X-Amz-Expires=300&X-Amz-Credential=ASIAQ3PHCVTYRBGSTTXK%2F20200111%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Signature=f3a0fb03947cf2be7f5c0d563d999a97cbc798dfcf6a9362970a2b4f22429f84&hash=733b1d2a78272d2393866d8fc6492b461308206ea979170d41dd5fa61f0ff15f&host=68042c943591013ac2b2430a89b270f6af2c76d8dfd086a07176afe7c76c2c61&pii=S0168010216302176&tid=spdf-83ad8d7e-b6c3-48f3-ac67-86f9a916e1d5&sid=45412b395b1d0047476b49a0dd1ef07e3ef6gxrqb&type=client https://www.sciencedirect.com/science/article/pii/S2211124715015375 https://www.sciencedirect.com/science/article/abs/pii/S0959438810001364#! https://www.karger.com/Article/FullText/341252 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6160412/ https://www.uniprot.org/uniprot/O43300 https://books.google.fr/books?id=hLS9BAAAQBAJ&pg=PA325&lpg=PA325&dq=LRRTM2+cytoplasmic+domain&source=bl&ots=5qlA_emVJs&sig=ACfU3U3tO8IN9lWW0rq3DDJbolvaJAo6Tw&hl=fr&sa=X&ved=2ahUKEwij9K_eofLmAhVCqxoKHZzPD8oQ6AEwAnoECAsQAQ#v=onepage&q=LRRTM2%20cytoplasmic%20domain&f=false - Cell Adhesion Molecules: Implications in Neurological Diseases publié par Vladimir Berezin, Peter S. Walmod https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3887770/ https://onlinelibrary.wiley.com/doi/pdf/10.1111/jnc.13159 https://www.rcsb.org/structure/5Z8X