We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1616
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | |
| - | == | + | ==bd Oxidase, 5DOQ== |
| - | <StructureSection load=' | + | <StructureSection load='5DOQ' size='350' frame='true' side='right' caption='bd Oxidase, 5DOQ' scene=’ |
| - | + | <Residues_22-44_of_bd_oxidase/2'> | |
| - | + | ||
| - | == | + | ==Introduction== |
| + | bd Oxidase is a type of quinol-dependent terminal oxidase found exclusively in prokaryotes. With a very high oxygen affinity, bd oxidases play a vital role in the oxidative phosphorylation pathway in both gram-positive and gram-negative bacteria. bd oxidases responsibility in the oxidative phosphorylation pathway allows the protein to also assist as a key survival factor in the bacterial stress response against antibacterial drugs. Given this knowledge, bd oxidases have become an area of scientific research worth pursuing as they could serve as an ideal target for antimicrobial drug development. | ||
| + | [[Image:proton graadient.jpg|300 px|left|thumb|Figure 1: Overall schematic representation of cytochrome bd; General display of the reduction of molecular oxygen into water [https://doi.org/10.1016/j.bbabio.2014.01.016.]]] | ||
| + | {{Clear}} | ||
| + | |||
| + | The overall mechanism of bd oxidases involves an exergonic reduction reaction of molecular oxygen into water. During this reaction, a proton gradient is generated in order to assist in the conservation of energy. Unlike other terminal oxidases, bd oxidases do not use a proton pump. Instead, bd oxidases use a form of vectorial chemistry that releases protons from the quinol oxidation into the positive, periplasmic side of the membrane. Protons that are required for the water formation are then consumed from the negative, cytoplasmic side of the membrane, thus creating the previously mentioned proton gradient. | ||
| + | |||
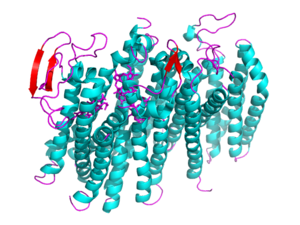
| + | [[Image: 5doq_WHOLE_IMAGE.png|300 px|left|thumb|Figure 2: 5DOQ monomer subunit; alpha helices in teal, beta sheets in purple.]] | ||
| + | {{Clear}} | ||
| + | This page will be specifically focusing on the structure and overall function of the 5DOQ bd oxidase. 5DOQ is a part of the long(L) quinol-binding domain subfamily that terminal oxidases are classified into. The L-subfamily of bd oxidases are responsible for the survival of acute infectious diseases such as E.Coli and salmonella. The 5DOQ's three heme groups, its periplasmically exposed Q loop, and four protein subunits will be of primary focus when identifying the relationship between structure and function. | ||
| + | |||
| + | == Function == | ||
| + | I am using<ref name="Safarian" /> this text to insert an in-text citation<ref name="Ransey" /> | ||
== Disease == | == Disease == | ||
| Line 12: | Line 23: | ||
== Structural highlights == | == Structural highlights == | ||
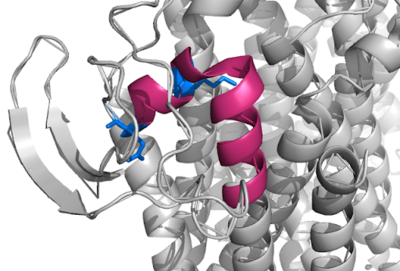
| - | + | [[Image:Q Loop.png|400 px|right|thumb|Figure 1]] | |
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| + | |||
| + | <scene name='83/837229/Residues_22-44_of_bd_oxidase/2'>bd Oxidase Residues 22-44</scene> | ||
| + | |||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | <ref name="Ransey">PMID:28504306</ref>. | ||
| + | <ref name="Safarian">PMID:27126043</ref>. | ||
<references/> | <references/> | ||
Revision as of 02:42, 31 March 2020
bd Oxidase, 5DOQ
| |||||||||||
References
- ↑ 1.0 1.1 Safarian S, Rajendran C, Muller H, Preu J, Langer JD, Ovchinnikov S, Hirose T, Kusumoto T, Sakamoto J, Michel H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science. 2016 Apr 29;352(6285):583-6. doi: 10.1126/science.aaf2477. PMID:27126043 doi:http://dx.doi.org/10.1126/science.aaf2477
- ↑ 2.0 2.1 Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
![Figure 1: Overall schematic representation of cytochrome bd; General display of the reduction of molecular oxygen into water [1]](/wiki/images/thumb/e/e6/Proton_graadient.jpg/300px-Proton_graadient.jpg)