We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1607
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==Introduction== | ==Introduction== | ||
| - | == | + | Calcium is a very important signaling molecule in the body with many physiological functions including muscle contraction, neuron excitability, cell migration and growth. The mitochondria are important regulators of calcium in the body and the calcium uniporter (MCU) maintains calcium homeostasis within the mitochondria. Calcium moves in one direction from the intermembrane space through the inner mitochondrial membrane into the matrix. The matrix is more negative driven by the respiratory chain which draws calcium in and allows calcium to move down its gradient. |
| + | The MCU is a complex. Its MICU1 and MICU2 bind together and associate with EMRE which regulates MCU. The MICU1 and MICU2 act as gatekeepers. EMRE connects the MICU1 and MICU2 sensors to MCU therefore regulating calcium uptake for the protein | ||
| + | |||
| + | The selectivity pore is an integral part of the protein. This pore contains a group of glutamate with oxygen facing inward forming a carboxylate ring through which calcium enters. This negative carboxylate ring does a good job of pulling the positive calcium into the selectivity pore at the top of the protein. | ||
| + | |||
| + | == Structural highlights and mechanism == | ||
| + | |||
| + | [[Image:Bubble_pic.png|300 px|right|thumb|Figure 2 Representation of the calcium fitting into the selectivity pore.]] | ||
| + | |||
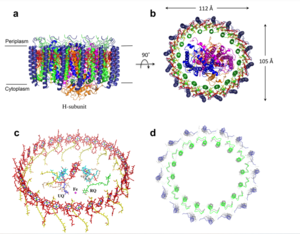
| + | The MCU is a dimer of dimers, described as tetrameric truncated pyramid. The uniporter has only a single strong binding site located in the selectivity pore, near the surface of the inner mitochondrial membrane. Activity of the uniporter is dependent on membrane potential and calcium concentration. Calcium from the cytoplasm enters the mitochondrial innermemnrane space through the mitochondrial membrane and is passed to the mitochondrial matrix via the MCU. [[Image:structure.png|300 px|right|thumb|Figure 2]] | ||
| + | |||
| + | ===Transmembrane Domain=== | ||
| + | The <scene name='83/837230/Selectivity_pore_space_fill/2'>Small pore, highly specific for calcium binding</scene> is located in the <scene name='83/837230/Transmembrane_domain/1'>transmembrane domain</scene>, in TM2 (transmembrane 2) while TM 1 (transmembrane 1) surrounds the pore. The transmembrane domain exhibits four fold rotational symmetry. The domain swapping of TM1 of one subunit tightly packing with the TM2 of the neighboring subunits. | ||
| + | |||
| + | ===Coiled coil=== | ||
| + | |||
| + | ===N-terminal Domain=== | ||
===Selectivity Filter=== | ===Selectivity Filter=== | ||
Revision as of 02:07, 31 March 2020
Mitochondrial Calcium Uniporter
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science. 2018 Jun 28. pii: science.aar4056. doi: 10.1126/science.aar4056. PMID:29954988 doi:http://dx.doi.org/10.1126/science.aar4056