Sandbox Reserved 1606
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Structural highlights == | == Structural highlights == | ||
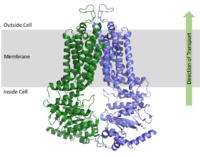
| - | ABCG2 is a homodimer with each dimer containing two domains, the nucleotide binding domain (NBD) and the transmembrane domain (TMD) which are fused together as a single peptide chain.<ref name="Taylor"/> The NBD is located inside of the cell and exposed to the cytosol while the TMD is embedded in the cell membrane and exposed to the extracellular space. [[Image:membrane domains.png|200 px|right|thumb|Figure 1. Domains of ABCG2 Multidrug transporter]] | + | ABCG2 is a homodimer with each dimer containing two domains, the nucleotide binding domain (NBD) and the transmembrane domain (TMD) which are fused together as a single peptide chain.<ref name="Taylor"/> The NBD is located inside of the cell and exposed to the cytosol while the TMD is embedded in the cell membrane and exposed to the extracellular space. [[Image:membrane domains.png|200 px|right|thumb|Figure 1. Domains of ABCG2 Multidrug transporter. The above protein is in the open, unbound ATP conformation]] |
===ATP Bound and Unbound Conformations=== | ===ATP Bound and Unbound Conformations=== | ||
As an [https://en.wikipedia.org/wiki/ATP-binding_cassette_transporter ABC Transporter], ABCG2 exhibits ATPase activity in which the protein binds and hydrolyzes ATP. After the binding of a substrate, one molecule of <scene name='83/832932/Atp_bound_use2/1'>ATP binds each NBD</scene> causing a conformational change of the overall structure from an <scene name='83/832932/Overall_structure/2'>open conformation</scene> with no ATP bound to a <scene name='83/832932/Atp_bound_use/1'>closed conformation</scene>. One molecule of ATP is hydrolyzed to transport substrates across the cell membrane while second molecule of ATP is hydrolyzed to reset the transporter to its open conformation.<ref name="Robey"/> | As an [https://en.wikipedia.org/wiki/ATP-binding_cassette_transporter ABC Transporter], ABCG2 exhibits ATPase activity in which the protein binds and hydrolyzes ATP. After the binding of a substrate, one molecule of <scene name='83/832932/Atp_bound_use2/1'>ATP binds each NBD</scene> causing a conformational change of the overall structure from an <scene name='83/832932/Overall_structure/2'>open conformation</scene> with no ATP bound to a <scene name='83/832932/Atp_bound_use/1'>closed conformation</scene>. One molecule of ATP is hydrolyzed to transport substrates across the cell membrane while second molecule of ATP is hydrolyzed to reset the transporter to its open conformation.<ref name="Robey"/> | ||
| Line 18: | Line 18: | ||
===Cavities and Leucine Plug=== | ===Cavities and Leucine Plug=== | ||
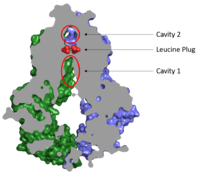
| - | [[Image:Map_leucine_plug.png|200 px|right|thumb|Figure 2. Locations of Cavities 1 and 2 and the Leucine Plug in ABCG2.]] | + | [[Image:Map_leucine_plug.png|200 px|right|thumb|Figure 2. Locations of Cavities 1 and 2 and the Leucine Plug in ABCG2. The above protein is in the open, unbound ATP conformation.]] |
Cavity 1 acts as the multidrug binding pocket | Cavity 1 acts as the multidrug binding pocket | ||
Revision as of 22:21, 4 April 2020
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
ABCG2 Multidrug Transporter
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ 2.0 2.1 2.2 Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ 3.0 3.1 Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018 Jul;18(7):452-464. doi: 10.1038/s41568-018-0005-8. PMID:29643473 doi:http://dx.doi.org/10.1038/s41568-018-0005-8
Student Contributors
Julia Pomeroy