We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Madison Summers/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
===Transmembrane Domain=== | ===Transmembrane Domain=== | ||
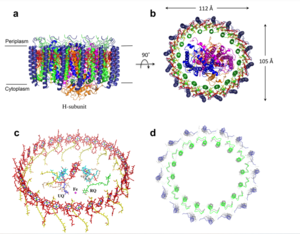
| - | The transmembrane domain is on the inner mitochondrial membrane open to the inner membrane space. The small pore, highly specific for calcium binding is located in the <scene name='83/837230/Transmembrane_domain/2'>transmembrane domain</scene>, in TM2 (transmembrane 2) while TM 1 (transmembrane 1) surrounds the pore. The transmembrane domain exhibits four fold rotational symmetry. The domain swapping of TM1 of one subunit with the TM2 of the neighboring subunits allows for a tight packing in the transmembrane connectivity. It is important that the selectivity pore is small, allowing only a dehydrated calcium molecule to interact with the 5 ampier wide glutamate ring. The negative charge of the glutamate's carboxyl group attracts the positively charged Calcium ion. Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12 ampier wide pore allowing high conductivity. The wider opening allows calcium to rehydrate once they pass the selectivity pore. Connectivity between subunits provide flexibility of the uniporter. | + | The transmembrane domain is on the [https://en.wikipedia.org/wiki/Mitochondrion#Structure inner mitochondrial membrane] open to the inner membrane space. The small pore, highly specific for calcium binding is located in the <scene name='83/837230/Transmembrane_domain/2'>transmembrane domain</scene>, in TM2 (transmembrane 2) while TM 1 (transmembrane 1) surrounds the pore. The transmembrane domain exhibits four fold rotational symmetry. The domain swapping of TM1 of one subunit with the TM2 of the neighboring subunits allows for a tight packing in the transmembrane connectivity. It is important that the selectivity pore is small, allowing only a dehydrated calcium molecule to interact with the 5 ampier wide glutamate ring. The negative charge of the glutamate's carboxyl group attracts the positively charged Calcium ion. Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12 ampier wide pore allowing high conductivity. The wider opening allows calcium to rehydrate once they pass the selectivity pore. Connectivity between subunits provide flexibility of the uniporter. |
===Soluble Domain=== | ===Soluble Domain=== | ||
The coiled coil is the first subsection of the soluble domain, which resides in the inner mitochondrial membrane. The coiled coil functions as the joints of the uniporter, providing flexibility to promote transport of Calcium ions down their concentration gradient. When calcium binds to the selectivity pore, the coiled coil swings approximately 8 degrees around its end near the N-terminal Domain. This movement propagates to the top of the transmembrane domain, where the pore is located, about 85 amperes away. The largest displacement triggered by the movement of the coiled coil is in the transmembrane domain, where the coil bends 20 degrees, moving the transmembrane domain further apart. | The coiled coil is the first subsection of the soluble domain, which resides in the inner mitochondrial membrane. The coiled coil functions as the joints of the uniporter, providing flexibility to promote transport of Calcium ions down their concentration gradient. When calcium binds to the selectivity pore, the coiled coil swings approximately 8 degrees around its end near the N-terminal Domain. This movement propagates to the top of the transmembrane domain, where the pore is located, about 85 amperes away. The largest displacement triggered by the movement of the coiled coil is in the transmembrane domain, where the coil bends 20 degrees, moving the transmembrane domain further apart. | ||
Revision as of 21:44, 6 April 2020
Mitochondrial Calcium Uniporter, E. coli
| |||||||||||
References
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science. 2018 Jun 28. pii: science.aar4056. doi: 10.1126/science.aar4056. PMID:29954988 doi:http://dx.doi.org/10.1126/science.aar4056
Student Contributors
- Madison Summers
- Holly Rowe
- Lizzy Ratz