We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | ='''Gamma Secretase'''= | + | <scene name='83/832945/Gluandtyr_remake/1'>Text To Be Displayed</scene>='''Gamma Secretase'''= |
<StructureSection load='5FN2' size='350' frame='true' side='right' caption='Human Gamma Secretase Basic Structure.' scene=’’> | <StructureSection load='5FN2' size='350' frame='true' side='right' caption='Human Gamma Secretase Basic Structure.' scene=’’> | ||
| Line 23: | Line 23: | ||
===Lid Complex=== | ===Lid Complex=== | ||
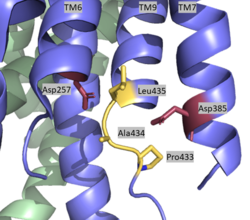
| - | + | The <scene name='83/832945/The_lid/1'>lid complex</scene> is the first point of entry and recognition for the substrate. This lid is located within the NCT subunit between Asn55 and Asn435. This lobe is divided into two separate subunits, and Phe287 from large lobe acts as pivot between them. This Phe is further surrounded by Phe103, Leu171, Phe176, and Ile180 of the small subunit, and these residues compose a greasy pocket for that provides an environment for easy movement. The lid consists of 5 aromatic residues which are highly involved with stabilizing the closed conformation. In particular, this conformation is stabilized by <scene name='83/832945/Thelid2/1'>Trp164, which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420</scene>. Once the substrate binds and the lid is opened, a charged, hydrophilic pocket is revealed. This pocket contains <scene name='83/832945/Gluandtyr_remake/1'>Glu333 and Tyr337 surrounded by several charged residues</scene>, and is involved with further substrate binding and recognition once the lid is removed. | |
| - | The <scene name='83/832945/The_lid/1'>lid complex</scene> is the first point of entry and recognition for the substrate. This lid is located within the NCT subunit between Asn55 and Asn435. This lobe is divided into two separate subunits, and Phe287 from large lobe acts as pivot between them. This Phe is further surrounded by Phe103, Leu171, Phe176, and Ile180 of the small subunit, and these residues compose a greasy pocket for that provides an environment for easy movement. The lid consists of 5 aromatic residues which are highly involved with stabilizing the closed conformation. In particular, this conformation is stabilized by <scene name='83/832945/Thelid2/1'>Trp164, which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420</scene>. Once the substrate binds and the lid is opened, a charged, hydrophilic pocket is revealed. This pocket contains <scene name='83/832945/ | + | |
Revision as of 03:32, 7 April 2020
=Gamma Secretase=
| |||||||||||
References
- ↑ Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
- ↑ Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
Student Contributors
Layla Wisser
Daniel Mulawa