We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1625
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
[[Image:Transmem1.png|300 px|right|thumb|'''Figure 2''. Cartoon model of cytochrome bd-oxidase in ''E. coli''. Dashed lines represent borders of cytoplasmic and extracellular regions.]] | [[Image:Transmem1.png|300 px|right|thumb|'''Figure 2''. Cartoon model of cytochrome bd-oxidase in ''E. coli''. Dashed lines represent borders of cytoplasmic and extracellular regions.]] | ||
| - | bd Oxidase is a type of quinol-dependent terminal oxidase found exclusively in prokaryotes.<ref name="Safarian">PMID: 27126043</ref> With a very high oxygen affinity, bd oxidases play a vital role in the oxidative phosphorylation pathway in both gram-positive and gram-negative bacteria. bd oxidases responsibility in the oxidative phosphorylation pathway allows the protein to also assist as a key survival factor in the bacterial stress response against antibacterial drugs. Given this knowledge, bd oxidases have become an area of scientific research worth pursuing as they could serve as an ideal target for antimicrobial drug development. | + | bd Oxidase is a type of quinol-dependent terminal oxidase found exclusively in prokaryotes.<ref name="Safarian">PMID: 27126043</ref> With a very high oxygen affinity, bd oxidases play a vital role in the oxidative phosphorylation pathway in both gram-positive and gram-negative bacteria. bd oxidases responsibility in the oxidative phosphorylation pathway allows the protein to also assist as a key survival factor in the bacterial stress response against antibacterial drugs. The <scene name='83/832931/Full/4'>cytochrome ''bd'' oxidase</scene> allows bacteria to be resistant to hypoxia, cyanide, nitric oxide, and H<sub>2</sub>O<sub>2</sub><ref name="Harikishore">PMID: 31939065</ref> |
| + | Given this knowledge, bd oxidases have become an area of scientific research worth pursuing as they could serve as an ideal target for antimicrobial drug development. | ||
[[Image:proton graadient.jpg|300 px|left|thumb|Figure 1: Overall schematic representation of cytochrome bd [https://doi.org/10.1016/j.bbabio.2014.01.016.]; General display of the reduction of molecular oxygen into water using the quinol as a reducing substrate. The three hemes are located near the periplasmic space, meaning that the membrane potential is generated mainly from proton transfer from the cytoplasm towards the active site on the opposite site of the membrane. Heme''b558'' is involved in quinol oxidation and Heme''d'' serves as the site where O2 binds and becomes reduced to H2O.]] | [[Image:proton graadient.jpg|300 px|left|thumb|Figure 1: Overall schematic representation of cytochrome bd [https://doi.org/10.1016/j.bbabio.2014.01.016.]; General display of the reduction of molecular oxygen into water using the quinol as a reducing substrate. The three hemes are located near the periplasmic space, meaning that the membrane potential is generated mainly from proton transfer from the cytoplasm towards the active site on the opposite site of the membrane. Heme''b558'' is involved in quinol oxidation and Heme''d'' serves as the site where O2 binds and becomes reduced to H2O.]] | ||
| Line 13: | Line 14: | ||
This page will be specifically focusing on the structure and overall function of the 6RX4 bd oxidase. 6RX4 is a part of the long(L) quinol-binding domain subfamily that terminal oxidases are classified into. The L-subfamily of bd oxidases are responsible for the survival of acute infectious diseases such as E.Coli and salmonella. The 6RX4's three <scene name='83/832931/Heme/4'>heme</scene> groups, its periplasmically exposed <scene name='83/832924/Q_loop/3'>Q-loop</scene>, and <scene name='83/832942/Four_subunits_labelled_6rx4/2'>four protein subunits</scene> will be of primary focus when identifying the relationship between structure and function. | This page will be specifically focusing on the structure and overall function of the 6RX4 bd oxidase. 6RX4 is a part of the long(L) quinol-binding domain subfamily that terminal oxidases are classified into. The L-subfamily of bd oxidases are responsible for the survival of acute infectious diseases such as E.Coli and salmonella. The 6RX4's three <scene name='83/832931/Heme/4'>heme</scene> groups, its periplasmically exposed <scene name='83/832924/Q_loop/3'>Q-loop</scene>, and <scene name='83/832942/Four_subunits_labelled_6rx4/2'>four protein subunits</scene> will be of primary focus when identifying the relationship between structure and function. | ||
| - | The <scene name='83/832931/Full/4'>cytochrome ''bd'' oxidase</scene> allows bacteria to be resistant to hypoxia, cyanide, nitric oxide, and H<sub>2</sub>O<sub>2</sub><ref name="Harikishore">PMID: 31939065</ref> | ||
==Structure== | ==Structure== | ||
Revision as of 04:42, 7 April 2020
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
Cytochrome bd-1 oxidase in Escherichia coli
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Safarian S, Rajendran C, Muller H, Preu J, Langer JD, Ovchinnikov S, Hirose T, Kusumoto T, Sakamoto J, Michel H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science. 2016 Apr 29;352(6285):583-6. doi: 10.1126/science.aaf2477. PMID:27126043 doi:http://dx.doi.org/10.1126/science.aaf2477
- ↑ 2.0 2.1 Harikishore A, Chong SSM, Ragunathan P, Bates RW, Gruber G. Targeting the menaquinol binding loop of mycobacterial cytochrome bd oxidase. Mol Divers. 2020 Jan 14. pii: 10.1007/s11030-020-10034-0. doi:, 10.1007/s11030-020-10034-0. PMID:31939065 doi:http://dx.doi.org/10.1007/s11030-020-10034-0
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Thesseling A, Rasmussen T, Burschel S, Wohlwend D, Kagi J, Muller R, Bottcher B, Friedrich T. Homologous bd oxidases share the same architecture but differ in mechanism. Nat Commun. 2019 Nov 13;10(1):5138. doi: 10.1038/s41467-019-13122-4. PMID:31723136 doi:http://dx.doi.org/10.1038/s41467-019-13122-4
- ↑ 4.0 4.1 4.2 4.3 4.4 Safarian S, Rajendran C, Muller H, Preu J, Langer JD, Ovchinnikov S, Hirose T, Kusumoto T, Sakamoto J, Michel H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science. 2016 Apr 29;352(6285):583-6. doi: 10.1126/science.aaf2477. PMID:27126043 doi:http://dx.doi.org/10.1126/science.aaf2477
- ↑ 5.0 5.1 Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Buttner L, Smoot MP, Behrendt CL, Cherry S, Santos RL, Hooper LV, Winter SE. Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe. 2017 Feb 8;21(2):208-219. doi: 10.1016/j.chom.2017.01.005. PMID:28182951 doi:http://dx.doi.org/10.1016/j.chom.2017.01.005
- ↑ 6.0 6.1 Shepherd M, Achard ME, Idris A, Totsika M, Phan MD, Peters KM, Sarkar S, Ribeiro CA, Holyoake LV, Ladakis D, Ulett GC, Sweet MJ, Poole RK, McEwan AG, Schembri MA. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Sci Rep. 2016 Oct 21;6:35285. doi: 10.1038/srep35285. PMID:27767067 doi:http://dx.doi.org/10.1038/srep35285
- ↑ Arora K, Ochoa-Montano B, Tsang PS, Blundell TL, Dawes SS, Mizrahi V, Bayliss T, Mackenzie CJ, Cleghorn LA, Ray PC, Wyatt PG, Uh E, Lee J, Barry CE 3rd, Boshoff HI. Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014 Nov;58(11):6962-5. doi: 10.1128/AAC.03486-14., Epub 2014 Aug 25. PMID:25155596 doi:http://dx.doi.org/10.1128/AAC.03486-14
- ↑ Galvan AE, Chalon MC, Rios Colombo NS, Schurig-Briccio LA, Sosa-Padilla B, Gennis RB, Bellomio A. Microcin J25 inhibits ubiquinol oxidase activity of purified cytochrome bd-I from Escherichia coli. Biochimie. 2019 May;160:141-147. doi: 10.1016/j.biochi.2019.02.007. Epub 2019 Feb, 19. PMID:30790617 doi:http://dx.doi.org/10.1016/j.biochi.2019.02.007
- ↑ Lu P, Heineke MH, Koul A, Andries K, Cook GM, Lill H, van Spanning R, Bald D. The cytochrome bd-type quinol oxidase is important for survival of Mycobacterium smegmatis under peroxide and antibiotic-induced stress. Sci Rep. 2015 May 27;5:10333. doi: 10.1038/srep10333. PMID:26015371 doi:http://dx.doi.org/10.1038/srep10333
Student Contributors
- Grace Bassler
- Emily Neal
- Marisa Villarreal
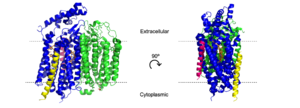
![Figure 1: Overall schematic representation of cytochrome bd [1]; General display of the reduction of molecular oxygen into water using the quinol as a reducing substrate. The three hemes are located near the periplasmic space, meaning that the membrane potential is generated mainly from proton transfer from the cytoplasm towards the active site on the opposite site of the membrane. Hemeb558 is involved in quinol oxidation and Hemed serves as the site where O2 binds and becomes reduced to H2O.](/wiki/images/thumb/e/e6/Proton_graadient.jpg/300px-Proton_graadient.jpg)

