We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
===Active Site=== | ===Active Site=== | ||
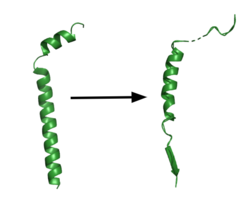
| - | The <scene name='83/832945/Asp_257_and_asp_385/ | + | The <scene name='83/832945/Asp_257_and_asp_385/11'>active site</scene> is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Each of these transmembrane helices has an aspartate residue, <scene name='83/832945/Asp_257_and_asp_385/10'>Asp257 and Asp385</scene>, which are located approximately 10.6 A˚ apart when inactive.<ref name="Bai">PMID:26280335</ref> The PAL sequence of <scene name='83/832945/Asp_257_and_asp_385/9'>Pro433, Ala434, and Leu435</scene> is in close proximity with the catalytic aspartates and is important to substrate recognition. GS becomes active upon substrate binding, when TM2 and TM6 each rotate about 15 degrees to more closely associate. Two β-strands are induced in PS1, creating an antiparallel β-sheet with the β-strand of APP.(B STRAND SOURCE The β-strand of the substrate interacts via main chain H-bonds with the PAL sequence, stabilizing the active site. Asp257 and Asp385 hydrogen bond to each other and are located 6–7 Å away from the scissile peptide bond of the substrate, allowing catalysis to occur.<ref name="Yang" /> The cleavage site is between the helix and the N-terminal β-strand of APP. GS cleaves in 3 residue segments which is driven by the presence of three amino acid binding pockets in the active site. GS can cleave via different pathways, depending on its starting point, but the 2 most commonly used pathways produce Aβ48 and Aβ49.<ref name="Bolduc">PMID:27580372</ref>. Tripeptide cleavage starting between Thr719 and Leu720 results in Aβ48. Cleavage between Leu720 and Val721 yields Aβ49.<ref name="Zhou">PMID:30630874</ref> |
Revision as of 03:25, 20 April 2020
Gamma Secretase
| |||||||||||
References
- ↑ 1.0 1.1 Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ 2.0 2.1 2.2 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
- ↑ Bolduc DM, Montagna DR, Seghers MC, Wolfe MS, Selkoe DJ. The amyloid-beta forming tripeptide cleavage mechanism of gamma-secretase. Elife. 2016 Aug 31;5. doi: 10.7554/eLife.17578. PMID:27580372 doi:http://dx.doi.org/10.7554/eLife.17578
- ↑ Kumar D, Ganeshpurkar A, Kumar D, Modi G, Gupta SK, Singh SK. Secretase inhibitors for the treatment of Alzheimer's disease: Long road ahead. Eur J Med Chem. 2018 Mar 25;148:436-452. doi: 10.1016/j.ejmech.2018.02.035. Epub , 2018 Feb 15. PMID:29477076 doi:http://dx.doi.org/10.1016/j.ejmech.2018.02.035
Student Contributors
Layla Wisser
Daniel Mulawa