We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
===Background=== | ===Background=== | ||
| - | Gamma secretase (GS) is a transmembrane [https://en.wikipedia.org/wiki/Aspartic_protease aspartatic protease] that catalyzes peptide bond hydrolysis of type I integral membrane proteins such as Notch, amyloid precursor protein (APP), and various other substrates. | + | Gamma secretase (GS) is a transmembrane [https://en.wikipedia.org/wiki/Aspartic_protease aspartatic protease] that catalyzes peptide bond hydrolysis of type I integral membrane proteins such as Notch, amyloid precursor protein (APP), and various other substrates. GS recognizes and catalyzes the cleavage of its substrate into 3 residue segments. Products of initial APP cleavage include the 48-residue peptide Aβ48 or the 49-residue peptide Aβ49. GS then cleaves these peptides into a variety of peptide fragments separated by 3 residues; Aβ49 is cleaved into Aβ46, Aβ43, and Aβ40; Aβ48 is cleaved into Aβ45, Aβ42, and Aβ38. These Aβ products are connected to neurological diseases like [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's disease (AD)] with varying length peptide products showing different disease symptoms. The connection between GS and AD has made a popular drug target. Various inhibitors of GS have been identified but no inhibitors have been clinically approved for treating AD, as the important neurological functions of gamma secretase has led to dangerous side effects upon inhibition. |
===Overall Structure=== | ===Overall Structure=== | ||
| Line 20: | Line 20: | ||
===Substrate Structure=== | ===Substrate Structure=== | ||
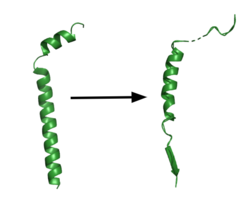
[[Image:App.png|250 px|right|thumb|'''Figure 1. APP fragment conformational change in gamma secretase.''' APP bound to GS undergoes a conformational change. The free state consists of 2 helices. Once bound to GS, the N-terminal helix unfolds into a coil and the C-terminal helix unwinds into a β-strand. This β-strand interacts with PS1 and is the site of cleavage by the protease.]] | [[Image:App.png|250 px|right|thumb|'''Figure 1. APP fragment conformational change in gamma secretase.''' APP bound to GS undergoes a conformational change. The free state consists of 2 helices. Once bound to GS, the N-terminal helix unfolds into a coil and the C-terminal helix unwinds into a β-strand. This β-strand interacts with PS1 and is the site of cleavage by the protease.]] | ||
| - | GS has been structurally characterized in the presence of both [https://en.wikipedia.org/wiki/Amyloid_precursor_protein/ APP] and Notch substrates. In each of these structures, the substrate bound in a similar location and underwent a similar structural transition upon binding to the active site of GS. Each substrate is composed of an N-terminal loop and a | + | GS has been structurally characterized in the presence of both [https://en.wikipedia.org/wiki/Amyloid_precursor_protein/ APP] and Notch substrates. In each of these structures, the substrate bound in a similar location and underwent a similar structural transition upon binding to the active site of GS. Each substrate is composed of an N-terminal loop and a TM helix. The peptide substrate enters the enzyme by <scene name='83/832945/App_in_gs_general/1'>lateral diffusion</scene> via the lid complex, and once in place, the TM helix of the substrate is anchored by <scene name='83/832945/Hydrophobic_interactions/2'>van der Waals contacts</scene>. Upon binding to GS, the C-terminal extracellular helix of the substrate unwinds. The substrate's N-terminal end of the TM helix unwinds into a β-strand (Fig. 1). To differentiate substrates, the β-strand is often the main point of identification for the enzyme. Substrate binding induces a structural change in GS, creating two β-strands that form a β-sheet with the one β-strand of the substrate. This β-sheet is in close proximity with the active site, and guides the process of catalysis.<ref name="Zhou">PMID:30630874</ref> |
| Line 34: | Line 34: | ||
==Relevance== | ==Relevance== | ||
| - | Gamma secretase | + | Gamma secretase is connected with the development of AD. In this, Aβ fragment build up leads to [https://en.wikipedia.org/wiki/Amyloid amyloid]plaques in brain.<ref name="Devendra">PMID:29477076</ref> These plaques then go on to cause severe neural dysfunction over time. |
| + | Mutations in GS are also connected with AD. Over 200 of GS mutations have been linked to causing AD. These mutations target "hot spots" on the enzyme and are aggregated at the interface between PS1 and APP.The vast majority of these mutations are clustered in regions surrounding the C-terminal half of the APP TM helix and the β-strand. Mutations at these locations affect the integrity of APP recruitment and catalysis, implicating a role in the development of Aβ plaques that impair neural function. In particular, Aβ42 and Aβ43 have implications with the formation of these plaques. | ||
| + | Inhibition of GS could be a potential AD treatment, but this would require targeting only APP cleavage over other GS substrates. The differential binding of APP and Notch to GS provides a starting point for this differentiation but will require further follow-up studies to confirm that the structural differences observed are biologically relevant. Currently, in order to combat this complex situation, differences in binding between different substrates are being utilized to create drugs that selectively inhibit APP binding with GS, and possibly create a more ideal target for AD treatment<ref name="Zhou">PMID:30630874</ref>. | ||
| + | |||
Revision as of 04:44, 21 April 2020
Gamma Secretase
| |||||||||||
References
- ↑ 1.0 1.1 Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ 2.0 2.1 2.2 2.3 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
- ↑ 4.0 4.1 Bolduc DM, Montagna DR, Seghers MC, Wolfe MS, Selkoe DJ. The amyloid-beta forming tripeptide cleavage mechanism of gamma-secretase. Elife. 2016 Aug 31;5. doi: 10.7554/eLife.17578. PMID:27580372 doi:http://dx.doi.org/10.7554/eLife.17578
- ↑ Kumar D, Ganeshpurkar A, Kumar D, Modi G, Gupta SK, Singh SK. Secretase inhibitors for the treatment of Alzheimer's disease: Long road ahead. Eur J Med Chem. 2018 Mar 25;148:436-452. doi: 10.1016/j.ejmech.2018.02.035. Epub , 2018 Feb 15. PMID:29477076 doi:http://dx.doi.org/10.1016/j.ejmech.2018.02.035
Student Contributors
Layla Wisser
Daniel Mulawa