We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
===Background=== | ===Background=== | ||
| - | Gamma secretase (GS) is a transmembrane [https://en.wikipedia.org/wiki/Aspartic_protease aspartatic protease] that catalyzes peptide bond hydrolysis of type I integral membrane proteins such as Notch, amyloid precursor protein (APP), and various other substrates. GS recognizes and catalyzes the cleavage of its substrate into 3 residue segments.<ref name="Bolduc" /> Products of initial APP cleavage include the 48-residue peptide Aβ48 or the 49-residue peptide Aβ49. GS then cleaves these peptides into a variety of peptide fragments separated by 3 residues; Aβ49 is cleaved into Aβ46, Aβ43, and Aβ40; Aβ48 is cleaved into Aβ45, Aβ42, and Aβ38. | + | Gamma secretase (GS) is a transmembrane [https://en.wikipedia.org/wiki/Aspartic_protease aspartatic protease] that catalyzes peptide bond hydrolysis of type I integral membrane proteins such as [https://en.wikipedia.org/wiki/Notch_signaling_pathway Notch], amyloid precursor protein (APP), and various other substrates. GS recognizes and catalyzes the cleavage of its substrate into 3 residue segments.<ref name="Bolduc" /> Products of initial APP cleavage include the 48-residue peptide Aβ48 or the 49-residue peptide Aβ49. GS then cleaves these peptides into a variety of peptide fragments separated by 3 residues; Aβ49 is cleaved into Aβ46, Aβ43, and Aβ40; Aβ48 is cleaved into Aβ45, Aβ42, and Aβ38. [https://en.wikipedia.org/wiki/Amyloid_beta Aβ products] are connected to neurological diseases such as [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's disease (AD)], with varying length peptide products showing different disease symptoms. The connection between GS and AD has made a popular drug target. Various inhibitors of GS have been identified, but no inhibitors have been clinically approved for treating AD. GS is linked to important neurological functions, leading to dangerous side effects upon inhibition<ref name="Zhou">PMID:30630874</ref>. |
===Overall Structure=== | ===Overall Structure=== | ||
GS is composed of 20 transmembrane components (TMs) and has 4 separate protein subunits: <font color='lightsteelblue'>Nicastran (NCT),</font> <font color='lightgreen'>Presenilin (PS1),</font> <font color= 'pink'>Anterior Pharynx-defective 1 (APH-1),</font> and <font color='khaki'>Presenilin Enhancer 2 (PEN-2).</font> These subunits are stabilized as functional GS by hydrophobic interactions and 4 [https://en.wikipedia.org/wiki/Phosphatidylcholine phosphatidylcholines].These <scene name='83/832945/Phosphotidylcholines/2'>phosphatidylcholines</scene> have interfaces between: PS1 and PEN-2, APH-1 and PS1, APH-1 and NCT. | GS is composed of 20 transmembrane components (TMs) and has 4 separate protein subunits: <font color='lightsteelblue'>Nicastran (NCT),</font> <font color='lightgreen'>Presenilin (PS1),</font> <font color= 'pink'>Anterior Pharynx-defective 1 (APH-1),</font> and <font color='khaki'>Presenilin Enhancer 2 (PEN-2).</font> These subunits are stabilized as functional GS by hydrophobic interactions and 4 [https://en.wikipedia.org/wiki/Phosphatidylcholine phosphatidylcholines].These <scene name='83/832945/Phosphotidylcholines/2'>phosphatidylcholines</scene> have interfaces between: PS1 and PEN-2, APH-1 and PS1, APH-1 and NCT. | ||
<scene name='83/832945/Nct_subunit_shown/1'>NCT</scene> has a large extracellular domain and one TM. NCT is important to substrate recognition and binding. | <scene name='83/832945/Nct_subunit_shown/1'>NCT</scene> has a large extracellular domain and one TM. NCT is important to substrate recognition and binding. | ||
| - | <scene name='83/832945/Ps1_subunit/1'>PS1</scene> serves as the active site of the protease and contains 9 TMs, each varying in length. The site of autocatalytic [https://en.wikipedia.org/wiki/Bond_cleavage cleavage] is located between <scene name='83/832945/Tm6_and_tm7/1'>TM6 and TM7</scene> of PS1 and a major conformational changes take place in | + | <scene name='83/832945/Ps1_subunit/1'>PS1</scene> serves as the active site of the protease and contains 9 TMs, each varying in length. The site of autocatalytic [https://en.wikipedia.org/wiki/Bond_cleavage cleavage] is located between <scene name='83/832945/Tm6_and_tm7/1'>TM6 and TM7</scene> of PS1 and a major conformational changes take place in the PS1 subunit upon substrate binding. |
<scene name='83/832945/Aph-1_subunit/1'>APH-1</scene> serves as a scaffold for anchoring and supporting the flexible conformational changes of PS1. | <scene name='83/832945/Aph-1_subunit/1'>APH-1</scene> serves as a scaffold for anchoring and supporting the flexible conformational changes of PS1. | ||
Activation of the active site is dependent on the binding of <scene name='83/832945/Pen2_subunit/1'>PEN-2</scene>. PEN-2 is also important in maturation of the enzyme.<ref name="Yang">PMID:28628788</ref> | Activation of the active site is dependent on the binding of <scene name='83/832945/Pen2_subunit/1'>PEN-2</scene>. PEN-2 is also important in maturation of the enzyme.<ref name="Yang">PMID:28628788</ref> | ||
| Line 19: | Line 19: | ||
== Structural highlights == | == Structural highlights == | ||
===Substrate Structure=== | ===Substrate Structure=== | ||
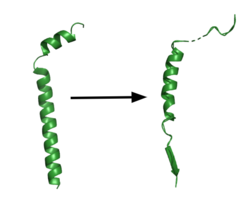
| - | [[Image:App.png|250 px|right|thumb|'''Figure 1. APP fragment conformational change in gamma secretase.''' APP bound to GS undergoes a conformational change. The free state consists of 2 helices. Once bound to GS, the N-terminal helix unfolds into a coil and the C-terminal helix unwinds into a β-strand. | + | [[Image:App.png|250 px|right|thumb|'''Figure 1. APP fragment conformational change in gamma secretase.''' APP bound to GS undergoes a conformational change. The free state consists of 2 helices. Once bound to GS, the N-terminal helix unfolds into a coil and the C-terminal helix unwinds into a β-strand. The β-strand of APP forms a β-sheet with PS1. Cleavage by the protease occurs between the helix and the β-strand.<ref name= "Zhou" />]] |
GS has been structurally characterized in the presence of both [https://en.wikipedia.org/wiki/Amyloid_precursor_protein/ APP] and Notch substrates. In each of these structures, the substrate bound in a similar location and underwent a similar structural transition upon binding to the active site of GS. Each substrate is composed of an N-terminal loop and a TM helix. The peptide substrate enters the enzyme by <scene name='83/832945/App_in_gs_general/1'>lateral diffusion</scene> via the lid complex, and once in place, the TM helix of the substrate is anchored by <scene name='83/832945/Hydrophobic_interactions/2'>van der Waals contacts</scene>. Upon binding to GS, the C-terminal extracellular helix of the substrate unwinds. The substrate's N-terminal end of the TM helix unwinds into a β-strand (Fig. 1). To differentiate substrates, the β-strand is often the main point of identification for the enzyme. Substrate binding induces a structural change in GS, creating two β-strands that form a β-sheet with the one β-strand of the substrate. This β-sheet is in close proximity with the active site, and guides the process of catalysis.<ref name="Zhou">PMID:30630874</ref> | GS has been structurally characterized in the presence of both [https://en.wikipedia.org/wiki/Amyloid_precursor_protein/ APP] and Notch substrates. In each of these structures, the substrate bound in a similar location and underwent a similar structural transition upon binding to the active site of GS. Each substrate is composed of an N-terminal loop and a TM helix. The peptide substrate enters the enzyme by <scene name='83/832945/App_in_gs_general/1'>lateral diffusion</scene> via the lid complex, and once in place, the TM helix of the substrate is anchored by <scene name='83/832945/Hydrophobic_interactions/2'>van der Waals contacts</scene>. Upon binding to GS, the C-terminal extracellular helix of the substrate unwinds. The substrate's N-terminal end of the TM helix unwinds into a β-strand (Fig. 1). To differentiate substrates, the β-strand is often the main point of identification for the enzyme. Substrate binding induces a structural change in GS, creating two β-strands that form a β-sheet with the one β-strand of the substrate. This β-sheet is in close proximity with the active site, and guides the process of catalysis.<ref name="Zhou">PMID:30630874</ref> | ||
===Lid Complex=== | ===Lid Complex=== | ||
| - | The <scene name='83/832945/Global_lid2/1'>lid complex</scene> is the first point of entry and recognition for the substrate. <scene name='83/832945/Lidremake2/1'> | + | The <scene name='83/832945/Global_lid2/1'>lid complex</scene> is the first point of entry and recognition for the substrate. <scene name='83/832945/Lidremake2/1'>The lid </scene> is located within the NCT subunit between Asn55 and Asn435. This lobe of NCT is divided into two separate subunits; the large and small lobes with Phe287 from the large lobe acting as a pivot between them. Phe287 is surrounded by <scene name='83/832945/Pivot3/1'>Phe103, Leu171, Phe176, and Ile180</scene> of the small subunit. The congregation of hydrophobic residues in the small subunit composes a greasy pocket which provides an environment for easy structural movement. The lid consists of 5 aromatic residues which are highly involved with stabilizing the closed conformation. In particular, this conformation is stabilized by <scene name='83/832945/Trp164scene/2'>Trp164, which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420</scene>. Once the substrate binds and the lid is opened, a charged, hydrophilic binding pocket is revealed. This pocket contains <scene name='83/832945/Gluandtyr_remake2/1'>Glu333 and Tyr337 surrounded by several charged residues</scene>, and is further involved with substrate binding and recognition once the lid is removed. However, this lid complex is relatively far away from the catalytic site of the enzyme in PS1. Once the substrate binds, the enzyme undergoes a conformational change to shorten this distance, and the rotation of the large lobe in relation to the small lobe reorients the substrate for cleavage by aligning the pocket in NCT to the active site in PS1. <ref name="Bai">PMID:26280335</ref> |
Revision as of 12:38, 21 April 2020
Gamma Secretase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Bolduc DM, Montagna DR, Seghers MC, Wolfe MS, Selkoe DJ. The amyloid-beta forming tripeptide cleavage mechanism of gamma-secretase. Elife. 2016 Aug 31;5. doi: 10.7554/eLife.17578. PMID:27580372 doi:http://dx.doi.org/10.7554/eLife.17578
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 3.2 Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ 4.0 4.1 4.2 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
- ↑ Bachurin SO, Bovina EV, Ustyugov AA. Drugs in Clinical Trials for Alzheimer's Disease: The Major Trends. Med Res Rev. 2017 Sep;37(5):1186-1225. doi: 10.1002/med.21434. Epub 2017 Jan 13. PMID:28084618 doi:http://dx.doi.org/10.1002/med.21434
- ↑ Kumar D, Ganeshpurkar A, Kumar D, Modi G, Gupta SK, Singh SK. Secretase inhibitors for the treatment of Alzheimer's disease: Long road ahead. Eur J Med Chem. 2018 Mar 25;148:436-452. doi: 10.1016/j.ejmech.2018.02.035. Epub , 2018 Feb 15. PMID:29477076 doi:http://dx.doi.org/10.1016/j.ejmech.2018.02.035
Student Contributors
Layla Wisser
Daniel Mulawa