We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
===Lid Complex=== | ===Lid Complex=== | ||
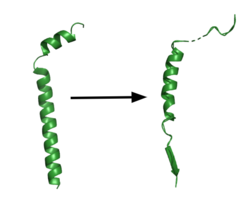
| - | The <scene name='83/832945/Global_lid2/1'>lid complex</scene> is the first point of entry and recognition for the substrate. <scene name='83/832945/Lidremake2/1'>The lid </scene> is located within the NCT subunit between Asn55 and Asn435. This lobe of NCT is divided into two separate subunits; the large and small lobes with Phe287 from the large lobe acting as a pivot between them. Phe287 is surrounded by <scene name='83/832945/Pivot3/1'>Phe103, Leu171, Phe176, and Ile180</scene> of the small subunit. The congregation of hydrophobic residues in the small subunit composes a greasy pocket which provides an environment for easy structural movement. The lid consists of 5 aromatic residues which are highly involved with stabilizing the closed conformation. | + | The <scene name='83/832945/Global_lid2/1'>lid complex</scene> is the first point of entry and recognition for the substrate. <scene name='83/832945/Lidremake2/1'>The lid </scene> is located within the NCT subunit between Asn55 and Asn435. This lobe of NCT is divided into two separate subunits; the large and small lobes with Phe287 from the large lobe acting as a pivot between them. Phe287 is surrounded by <scene name='83/832945/Pivot3/1'>Phe103, Leu171, Phe176, and Ile180</scene> of the small subunit. The congregation of hydrophobic residues in the small subunit composes a greasy pocket which provides an environment for easy structural movement. The lid consists of 5 aromatic residues, which are highly involved with stabilizing the closed conformation. This conformation is stabilized by <scene name='83/832945/Trp164scene/2'>Trp164, which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420</scene>. Once the substrate binds and the lid is opened, a charged, hydrophilic binding pocket is revealed. The pocket contains <scene name='83/832945/Gluandtyr_remake2/1'>Glu333 and Tyr337 surrounded by several charged residues</scene>. The pocket is further involved with substrate binding and recognition once the lid is removed. The lid complex is relatively far away from the catalytic site of the enzyme in PS1 when inactive. Once a substrate binds, the enzyme undergoes a conformational change in which the rotation of the large lobe in relation to the small lobe reorients the substrate for cleavage, by aligning the pocket in NCT to the active site in PS1.<ref name="Bai">PMID:26280335</ref> |
Revision as of 12:44, 21 April 2020
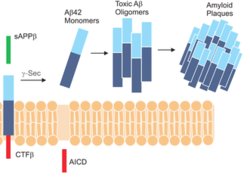
Gamma Secretase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Bolduc DM, Montagna DR, Seghers MC, Wolfe MS, Selkoe DJ. The amyloid-beta forming tripeptide cleavage mechanism of gamma-secretase. Elife. 2016 Aug 31;5. doi: 10.7554/eLife.17578. PMID:27580372 doi:http://dx.doi.org/10.7554/eLife.17578
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 3.2 Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ 4.0 4.1 4.2 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
- ↑ Bachurin SO, Bovina EV, Ustyugov AA. Drugs in Clinical Trials for Alzheimer's Disease: The Major Trends. Med Res Rev. 2017 Sep;37(5):1186-1225. doi: 10.1002/med.21434. Epub 2017 Jan 13. PMID:28084618 doi:http://dx.doi.org/10.1002/med.21434
- ↑ Kumar D, Ganeshpurkar A, Kumar D, Modi G, Gupta SK, Singh SK. Secretase inhibitors for the treatment of Alzheimer's disease: Long road ahead. Eur J Med Chem. 2018 Mar 25;148:436-452. doi: 10.1016/j.ejmech.2018.02.035. Epub , 2018 Feb 15. PMID:29477076 doi:http://dx.doi.org/10.1016/j.ejmech.2018.02.035
Student Contributors
Layla Wisser
Daniel Mulawa