This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 897
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Function == | == Function == | ||
| + | |||
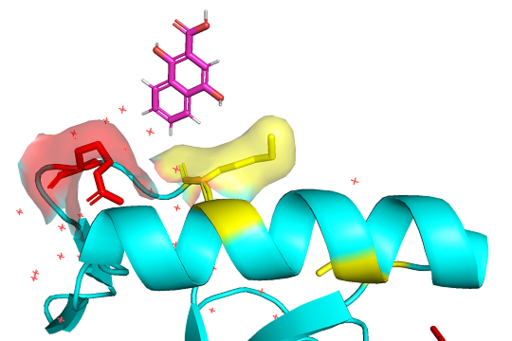
| + | ===Methyl-lysine reader=== | ||
| + | PWWP domains are one of epigenetic regulators which recognize specific lysine residues which are methylated. Even though there is no detailed research on 3pfs, it may be assumed to have similar function with BRPF1 PWWP domain which is shown to have methylated histone binding activity.<ref>Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Göttgens B, Bycroft M. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nature structural & molecular biology. 2010 May;17(5):617-9.</ref> With a high-throughput mass spectrometry screening, this motif is suggested as an essential motif of histone 3 lysine 36 trimethylation binding.<ref>Vermeulen M, Eberl HC, Matarese F, Marks H, Denissov S, Butter F, Lee KK, Olsen JV, Hyman AA, Stunnenberg HG, Mann M. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010 Sep 17;142(6):967-80.</ref> The β-strands and Pro-Trp-Trp-Pro motif form hydrophobic cavity, which is the binding site of methylated lysines.<ref>Rona GB, Eleutherio EC, Pinheiro AS. PWWP domains and their modes of sensing DNA and histone methylated lysines. Biophysical reviews. 2016 Mar 1;8(1):63-74.</ref> Since the aromatic cage is a common structure in the Royal family, it can be a common tool for methylated lysine binding.<ref>Qiu Y, Zhang W, Zhao C, Wang Y, Wang W, Zhang J, Zhang Z, Li G, Shi Y, Tu X, Wu J. Solution structure of the Pdp1 PWWP domain reveals its unique binding sites for methylated H4K20 and DNA. Biochemical Journal. 2012 Mar 15;442(3):527-38.</ref> | ||
===Nonspecific binding=== | ===Nonspecific binding=== | ||
| - | The PWWP domains have a significant amount of basic residues.<ref>Qiu C, Sawada K, Zhang X, Cheng X. The PWWP domain of mammalian DNA methyltransferase Dnmt3b defines a new family of DNA-binding folds. Nature structural biology. 2002 Mar;9(3):217-24.</ref> 3pfs | + | The PWWP domains have a significant amount of basic residues which gives ability of nonspecific binding on DNA strands.<ref>Qiu C, Sawada K, Zhang X, Cheng X. The PWWP domain of mammalian DNA methyltransferase Dnmt3b defines a new family of DNA-binding folds. Nature structural biology. 2002 Mar;9(3):217-24.</ref> BRPF3 3pfs protein has 11 lysine residues and 12 arginine residues. These residues' sidechains remain being positively charged on in vivo pH. The DNA strand phosphate backbone, which is negatively charged, would be attracted to PWWP domain surface due to the nature of attraction between the opposite charges. |
[[Image:3pfs non-selective model.png|thumb|center|512 px| '''Figure 2:''' Lysine(yellow) and Arginine(red) residues on 3PFS surface interacting with DNA backbone generated in PyMOL using PDB: ''3PFS'']] | [[Image:3pfs non-selective model.png|thumb|center|512 px| '''Figure 2:''' Lysine(yellow) and Arginine(red) residues on 3PFS surface interacting with DNA backbone generated in PyMOL using PDB: ''3PFS'']] | ||
| - | ===Methyl-lysine reader=== | ||
| - | PWWP domains are one of epigenetic regulators which recognize specific lysine residues which are methylated. Even though there is no detailed research on 3pfs, it is assumed to have similar function with BRPF1 PWWP domain which is shown to have methylated histone binding activity.<ref>Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Göttgens B, Bycroft M. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nature structural & molecular biology. 2010 May;17(5):617-9.</ref> With a high-throughput mass spectrometry screening, this motif is suggested as an essential motif of histone 3 lysine 36 trimethylation binding.<ref>Vermeulen M, Eberl HC, Matarese F, Marks H, Denissov S, Butter F, Lee KK, Olsen JV, Hyman AA, Stunnenberg HG, Mann M. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010 Sep 17;142(6):967-80.</ref> The β-strands and Pro-Trp-Trp-Pro motif form hydrophobic cavity, which is the binding site of methylated lysines.<ref>Rona GB, Eleutherio EC, Pinheiro AS. PWWP domains and their modes of sensing DNA and histone methylated lysines. Biophysical reviews. 2016 Mar 1;8(1):63-74.</ref> Since the aromatic cage is a common structure in the Royal family, it can be a common tool for methylated lysine binding.<ref>Qiu Y, Zhang W, Zhao C, Wang Y, Wang W, Zhang J, Zhang Z, Li G, Shi Y, Tu X, Wu J. Solution structure of the Pdp1 PWWP domain reveals its unique binding sites for methylated H4K20 and DNA. Biochemical Journal. 2012 Mar 15;442(3):527-38.</ref> | ||
| Line 24: | Line 25: | ||
== Therapheutic features == | == Therapheutic features == | ||
| - | There is no research on the relationship between BRPF3 PWWP domain and any human disease. However, its | + | There is no research on the relationship between BRPF3 PWWP domain and any human disease. However, both of its closely related domains BRPF1 and BRPF2 PWWP domains are associated with hox gene regulation, that is associated with schizophrenia and bipolar affective disorder.<ref>Bjarkam CR, Corydon TJ, Olsen IM, Pallesen J, Nyegaard M, Fryland T, Mors O, Børglum AD. Further immunohistochemical characterization of BRD1 a new susceptibility gene for schizophrenia and bipolar affective disorder. Brain Structure and Function. 2009 Dec 1;214(1):37.</ref> Therefore BRPF3 PWWP domain, which has very similar β-barrel structure with previous proteins, may also have Hox gene-related function. But a further research would be required. |
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
Revision as of 18:30, 28 April 2020
| This Sandbox is Reserved from Jan 13 through July 31, 2020 for use in the course Protein Structure in Drug Discovery taught by Karen C. Glass at the ACPHS, Colchester, United States. This reservation includes Sandbox Reserved 895 through Sandbox Reserved 901. |
To get started:
More help: Help:Editing |
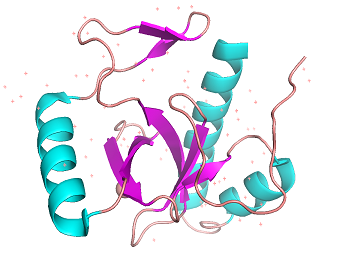
BRPF3 PWWP binding domain 3PFS
| |||||||||||
References
- ↑ Rona GB, Eleutherio EC, Pinheiro AS. PWWP domains and their modes of sensing DNA and histone methylated lysines. Biophysical reviews. 2016 Mar 1;8(1):63-74.
- ↑ Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Göttgens B, Bycroft M. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nature structural & molecular biology. 2010 May;17(5):617-9.
- ↑ Rona GB, Eleutherio EC, Pinheiro AS. PWWP domains and their modes of sensing DNA and histone methylated lysines. Biophysical reviews. 2016 Mar 1;8(1):63-74.
- ↑ Hung YL, Lee HJ, Jiang I, Lin SC, Lo WC, Lin YJ, Sue SC. The first residue of the PWWP motif modulates HATH domain binding, Stability, and Protein–Protein Interaction. Biochemistry. 2015 Jul 7;54(26):4063-74.
- ↑ Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Göttgens B, Bycroft M. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nature structural & molecular biology. 2010 May;17(5):617-9.
- ↑ Vermeulen M, Eberl HC, Matarese F, Marks H, Denissov S, Butter F, Lee KK, Olsen JV, Hyman AA, Stunnenberg HG, Mann M. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010 Sep 17;142(6):967-80.
- ↑ Rona GB, Eleutherio EC, Pinheiro AS. PWWP domains and their modes of sensing DNA and histone methylated lysines. Biophysical reviews. 2016 Mar 1;8(1):63-74.
- ↑ Qiu Y, Zhang W, Zhao C, Wang Y, Wang W, Zhang J, Zhang Z, Li G, Shi Y, Tu X, Wu J. Solution structure of the Pdp1 PWWP domain reveals its unique binding sites for methylated H4K20 and DNA. Biochemical Journal. 2012 Mar 15;442(3):527-38.
- ↑ Qiu C, Sawada K, Zhang X, Cheng X. The PWWP domain of mammalian DNA methyltransferase Dnmt3b defines a new family of DNA-binding folds. Nature structural biology. 2002 Mar;9(3):217-24.
- ↑ Rona GB, Eleutherio EC, Pinheiro AS. PWWP domains and their modes of sensing DNA and histone methylated lysines. Biophysical reviews. 2016 Mar 1;8(1):63-74.
- ↑ Bjarkam CR, Corydon TJ, Olsen IM, Pallesen J, Nyegaard M, Fryland T, Mors O, Børglum AD. Further immunohistochemical characterization of BRD1 a new susceptibility gene for schizophrenia and bipolar affective disorder. Brain Structure and Function. 2009 Dec 1;214(1):37.