This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Adenomatous polyposis coli
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== The overall structure of APC == | == The overall structure of APC == | ||
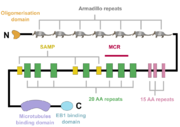
[[Image:APC.png|thumb|A schematic of the APC protein domain structure. MCR, mutation cluster region; SAMP, Axin-binding motif ]] | [[Image:APC.png|thumb|A schematic of the APC protein domain structure. MCR, mutation cluster region; SAMP, Axin-binding motif ]] | ||
| - | The APC protein, its primary sequence encompassing 2843 aminoacids<ref name="uniprot">https://www.uniprot.org/uniprot/P25054</ref>, consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise. It is followed by | + | The APC protein, its primary sequence encompassing 2843 aminoacids<ref name="uniprot">https://www.uniprot.org/uniprot/P25054</ref>, consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise. It is followed by so called pre-ARM region and seven armadillo repeats, which form a groove for binding of a guanine nucleotide exchange factor Asef<ref name="Zhang2012">Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.</ref>. The central part of APC contains three 15 aminoacid long repeats followed by seven 20 aminoacid long repeats<ref name="Zhang2017"/>. These motifs serve as binding sites for β-catenin<ref name="Hou2011">Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.</ref>. In between the 20 aminoacid repeats, three SAMP regions are dispersed, enabling the interaction with Axin<ref name="Zhang2017"/>. At the C-terminus, a basic domain responsible for binding to microtubules as well as EB1 interaction domain are present<ref name="Su1995">Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.</ref><ref name="Zhang2017"/>. |
Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513 <ref name="Miyoshi1992">Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)</ref>. | Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513 <ref name="Miyoshi1992">Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)</ref>. | ||
== The physiological functions of APC and their implications for colorectal cancer onset and progression == | == The physiological functions of APC and their implications for colorectal cancer onset and progression == | ||
| + | The seven armadillo repeats (ARM) together with the so-called pre-ARM region adjoining them at the N-terminus are essential for binding the guanine nucleotide exchange factor Asef<ref name="Zhang2012"/>. In the absence of APC, Asef adopts an autoinhibited conformation, which prevents it from interaction with the small GTPase Cdc42<ref name="Mitin2007">Mitin, N. et al. (2007) ‘Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression’, Nature Structural and Molecular Biology, 14(9), pp. 814–823. doi: 10.1038/nsmb1290.</ref>. Upon APC binding, the autoinhibited conformation of Asef is disrupted and the binding site for Cdc42 is made accessible<ref name="Zhang2012"/>. Interaction with Asef leads to the exchange of GDP for GTP in the Cdc42 protein, which in turn modulates adherent junctions and contributes to enhanced cell motility<ref name="Kawasaki2003">Kawasaki, Y., Sato, R. and Akiyama, T. (2003) ‘Mutated APC and Asef are involved in the migration of colorectal tumour cells’, Nature Cell Biology, 5(3), pp. 211–215. doi: 10.1038/ncb937.</ref><ref name="Mitin2007"/><ref name="Zhang2012"/>. In colorectal cancers, the truncated version of APC with preserved pre-ARM and ARM domains constitutively activates Asef and hence Cdc42<ref name="Kawasaki2010">Kawasaki, Y. et al. (2010) ‘The adenomatous polyposis coli-associated guanine nucleotide exchange factor Asef is involved in angiogenesis’, Journal of Biological Chemistry, 285(2), pp. 1199–1207. doi: 10.1074/jbc.M109.040691.</ref>. This leads to extracellular matrix remodelling and promotion of adhesion-independent growth and cell migration<ref name="Kawasaki2009">Kawasaki, Y. et al. (2009) ‘The adenomatous polyposis coli-associated exchange factors Asef and Asef2 are required for adenoma formation in ApcMin/+mice’, EMBO Reports, 10(12), pp. 1355–1362. doi: 10.1038/embor.2009.233.</ref>. | ||
| + | Interestingly, APC takes part in strengthening the adherent junctions through the regulation of cellular distribution of E-cadherin and β-catenin. Full-length APC leads to increased levels of E-cadherin at the plasma membrane and decreases the pool of nuclear β-catenin in favour of the cytosolic one, enabling adherent junctions to be formed<ref name="Faux2004">Faux, M. C. et al. (2004) ‘Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion’, Journal of Cell Science, 117(3), pp. 427–439. doi: 10.1242/jcs.00862.</ref>. On the other hand, the truncated form of APC lacking the β-catenin interaction motifs is unable of such actions<ref name="Kawasaki2003"/>. | ||
=== Regulation of cell adhesion and migration === | === Regulation of cell adhesion and migration === | ||
Revision as of 15:30, 29 April 2020
Adenomatous polyposis coli
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Zhang, L. and Shay, J. W. (2017) ‘Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.’, Journal of the National Cancer Institute, 109(8). doi: 10.1093/jnci/djw332.
- ↑ https://www.proteinatlas.org/ENSG00000134982-APC/tissue
- ↑ Ficari, F. et al. (2000) ‘APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis’, British Journal of Cancer. Churchill Livingstone, 82(2), pp. 348–353. doi: 10.1054/bjoc.1999.0925.
- ↑ Rowan, A. J. et al. (2000) ‘APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 97(7), pp. 3352–3357. doi: 10.1073/pnas.97.7.3352.
- ↑ https://www.uniprot.org/uniprot/P25054
- ↑ 6.0 6.1 6.2 6.3 Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.
- ↑ Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.
- ↑ Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.
- ↑ Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)
- ↑ 10.0 10.1 Mitin, N. et al. (2007) ‘Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression’, Nature Structural and Molecular Biology, 14(9), pp. 814–823. doi: 10.1038/nsmb1290.
- ↑ 11.0 11.1 Kawasaki, Y., Sato, R. and Akiyama, T. (2003) ‘Mutated APC and Asef are involved in the migration of colorectal tumour cells’, Nature Cell Biology, 5(3), pp. 211–215. doi: 10.1038/ncb937.
- ↑ Kawasaki, Y. et al. (2010) ‘The adenomatous polyposis coli-associated guanine nucleotide exchange factor Asef is involved in angiogenesis’, Journal of Biological Chemistry, 285(2), pp. 1199–1207. doi: 10.1074/jbc.M109.040691.
- ↑ Kawasaki, Y. et al. (2009) ‘The adenomatous polyposis coli-associated exchange factors Asef and Asef2 are required for adenoma formation in ApcMin/+mice’, EMBO Reports, 10(12), pp. 1355–1362. doi: 10.1038/embor.2009.233.
- ↑ Faux, M. C. et al. (2004) ‘Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion’, Journal of Cell Science, 117(3), pp. 427–439. doi: 10.1242/jcs.00862.