This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Adenomatous polyposis coli
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
'''<scene name='84/843011/Pokus1/1'>Adenomatous polyposis coli (APC)</scene>''' is a multidomain tumour suppressor protein involved in the regulation of various cellular processes, such as cell adhesion, migration or proliferation<ref name="Zhang2017">Zhang, L. and Shay, J. W. (2017) ‘Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.’, Journal of the National Cancer Institute, 109(8). doi: 10.1093/jnci/djw332.</ref>. It is expressed in plethora of organs and tissues, e. g. cerebral cortex, bronchi or the gastrointestinal tract<ref name="proteinatlas">https://www.proteinatlas.org/ENSG00000134982-APC/tissue</ref>. Germline truncation mutations of APC result in familial adenomatous polyposis, a hereditary form of colon cancer<ref name="Ficari2000">Ficari, F. et al. (2000) ‘APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis’, British Journal of Cancer. Churchill Livingstone, 82(2), pp. 348–353. doi: 10.1054/bjoc.1999.0925.</ref>. Additionally, loss of the C-terminal portion of APC is detected in about 80 % of sporadic colon cancers<ref name="Rowan2000">Rowan, A. J. et al. (2000) ‘APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 97(7), pp. 3352–3357. doi: 10.1073/pnas.97.7.3352.</ref>. | '''<scene name='84/843011/Pokus1/1'>Adenomatous polyposis coli (APC)</scene>''' is a multidomain tumour suppressor protein involved in the regulation of various cellular processes, such as cell adhesion, migration or proliferation<ref name="Zhang2017">Zhang, L. and Shay, J. W. (2017) ‘Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.’, Journal of the National Cancer Institute, 109(8). doi: 10.1093/jnci/djw332.</ref>. It is expressed in plethora of organs and tissues, e. g. cerebral cortex, bronchi or the gastrointestinal tract<ref name="proteinatlas">https://www.proteinatlas.org/ENSG00000134982-APC/tissue</ref>. Germline truncation mutations of APC result in familial adenomatous polyposis, a hereditary form of colon cancer<ref name="Ficari2000">Ficari, F. et al. (2000) ‘APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis’, British Journal of Cancer. Churchill Livingstone, 82(2), pp. 348–353. doi: 10.1054/bjoc.1999.0925.</ref>. Additionally, loss of the C-terminal portion of APC is detected in about 80 % of sporadic colon cancers<ref name="Rowan2000">Rowan, A. J. et al. (2000) ‘APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 97(7), pp. 3352–3357. doi: 10.1073/pnas.97.7.3352.</ref>. | ||
| + | |||
== The overall structure of APC == | == The overall structure of APC == | ||
| Line 7: | Line 8: | ||
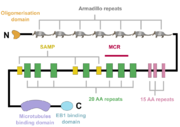
The APC protein, its primary sequence encompassing 2843 aminoacids<ref name="uniprot">https://www.uniprot.org/uniprot/P25054</ref>, consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise. It is followed by so called pre-ARM region and seven armadillo repeats, which form a groove for binding of a guanine nucleotide exchange factor Asef<ref name="Zhang2012">Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.</ref>. The central part of APC contains three 15 aminoacid long repeats followed by seven 20 aminoacid long repeats<ref name="Zhang2017"/>. These motifs serve as binding sites for β-catenin<ref name="Hou2011">Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.</ref>. In between the 20 aminoacid repeats, three SAMP regions are dispersed, enabling the interaction with Axin<ref name="Zhang2017"/>. At the C-terminus, a basic domain responsible for binding to microtubules as well as EB1 interaction domain are present<ref name="Su1995">Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.</ref><ref name="Zhang2017"/>. | The APC protein, its primary sequence encompassing 2843 aminoacids<ref name="uniprot">https://www.uniprot.org/uniprot/P25054</ref>, consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise. It is followed by so called pre-ARM region and seven armadillo repeats, which form a groove for binding of a guanine nucleotide exchange factor Asef<ref name="Zhang2012">Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.</ref>. The central part of APC contains three 15 aminoacid long repeats followed by seven 20 aminoacid long repeats<ref name="Zhang2017"/>. These motifs serve as binding sites for β-catenin<ref name="Hou2011">Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.</ref>. In between the 20 aminoacid repeats, three SAMP regions are dispersed, enabling the interaction with Axin<ref name="Zhang2017"/>. At the C-terminus, a basic domain responsible for binding to microtubules as well as EB1 interaction domain are present<ref name="Su1995">Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.</ref><ref name="Zhang2017"/>. | ||
Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513<ref name="Miyoshi1992">Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)</ref>. | Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513<ref name="Miyoshi1992">Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)</ref>. | ||
| + | |||
== The physiological functions of APC and their implications for colorectal cancer onset and progression == | == The physiological functions of APC and their implications for colorectal cancer onset and progression == | ||
| Line 22: | Line 24: | ||
=== Gain of function APC mutants === | === Gain of function APC mutants === | ||
In addition to loss-of-function mutants of APC which are not able to perform its tumour suppressive functions, a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a gain-of-function manner. Some studies even show that such mutant forms act in a dominant way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle<ref name="Tighe2004"/><ref name="Green2005"/>, antagonise the induction of apoptotic cell death<ref name="Qian2007">Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.</ref><ref name="Brocardo2008">Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.</ref>, enhance cell migration<ref name="Kawasaki2003"/> or compromise directional cell migration<ref name="Nelson2012">Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.</ref>. | In addition to loss-of-function mutants of APC which are not able to perform its tumour suppressive functions, a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a gain-of-function manner. Some studies even show that such mutant forms act in a dominant way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle<ref name="Tighe2004"/><ref name="Green2005"/>, antagonise the induction of apoptotic cell death<ref name="Qian2007">Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.</ref><ref name="Brocardo2008">Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.</ref>, enhance cell migration<ref name="Kawasaki2003"/> or compromise directional cell migration<ref name="Nelson2012">Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.</ref>. | ||
| + | |||
== Structural insights into APC interactions == | == Structural insights into APC interactions == | ||
| Line 28: | Line 31: | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 16:50, 29 April 2020
Adenomatous polyposis coli
| |||||||||||