User:Samantha Schneider/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
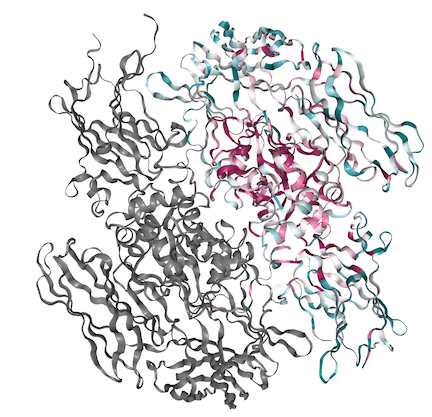
The fibrin stabilization factor is a heterotetramer that circulates throughout the blood plasma as a 320 kda molecule. It consists of a dimer of A subunits and a dimer of B subunits. The FXIIIA subunit is composed of 4 structural units: <scene name='84/842930/Beta_sandwhich/1'>Beta sandwich</scene>, core, <scene name='84/842930/B-barrel_1/1'>barrel-1</scene>, and barrel-2 domains. The A subunit has a 37 amino acid N-terminal activation peptide, this is cleaved by thrombin during FXIII activation to FXIIIa. The [https://en.wikipedia.org/wiki/Beta-sandwich beta sandwhich] consists of residues 38-184. The <scene name='84/842930/Activation/1'>activation peptide</scene> is the first 37 amino acids on the N-terminal of the A subunit<ref>Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.</ref>. | The fibrin stabilization factor is a heterotetramer that circulates throughout the blood plasma as a 320 kda molecule. It consists of a dimer of A subunits and a dimer of B subunits. The FXIIIA subunit is composed of 4 structural units: <scene name='84/842930/Beta_sandwhich/1'>Beta sandwich</scene>, core, <scene name='84/842930/B-barrel_1/1'>barrel-1</scene>, and barrel-2 domains. The A subunit has a 37 amino acid N-terminal activation peptide, this is cleaved by thrombin during FXIII activation to FXIIIa. The [https://en.wikipedia.org/wiki/Beta-sandwich beta sandwhich] consists of residues 38-184. The <scene name='84/842930/Activation/1'>activation peptide</scene> is the first 37 amino acids on the N-terminal of the A subunit<ref>Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.</ref>. | ||
| - | FXIIIB subunits are glycoproteins. The B subunit is made up of ten [https://en.wikipedia.org/wiki/Sushi_domain sushi] domains. The <scene name='84/842930/Sushi-1/1'>Sushi</scene> domains which are each composed of approximately 60 amino acids. The B subunit is known to have a protective role, but recent research has suggested that there may be a regulatory role as well. The Sushi domain's variable length loop region is shown to have a hydrophobic interaction with the N-terminal activation region of the A subunit. The variable length loop region of the sushi-1 domain is electrostatically neutral. The Cab1 site is always exposed and therefore is bound by a calcium ion | + | FXIIIB subunits are glycoproteins. The B subunit is made up of ten [https://en.wikipedia.org/wiki/Sushi_domain sushi] domains. The <scene name='84/842930/Sushi-1/1'>Sushi</scene> domains which are each composed of approximately 60 amino acids. The B subunit is known to have a protective role, but recent research has suggested that there may be a regulatory role as well. The Sushi domain's variable length loop region is shown to have a hydrophobic interaction with the N-terminal activation region of the A subunit. The variable length loop region of the sushi-1 domain is electrostatically neutral. The Cab1 site is always exposed and therefore is bound by a calcium ion. |
<scene name='84/842930/Hydrophobic_tunnel/1'>Hydrophobic Tunnel</scene> is formed in the A subunit upon activation of molecule by calcium. It is the entry for the Q and K substrate to the binding site. It is formed by planar interactions of the Trp rings. The Cysteine is reactive in the binding pocket. | <scene name='84/842930/Hydrophobic_tunnel/1'>Hydrophobic Tunnel</scene> is formed in the A subunit upon activation of molecule by calcium. It is the entry for the Q and K substrate to the binding site. It is formed by planar interactions of the Trp rings. The Cysteine is reactive in the binding pocket. | ||
| Line 23: | Line 23: | ||
== Disease == | == Disease == | ||
| - | Factor XIII deficiency is a genetic bleeding disorder. It is an autosomal recessive disease. It is rare with Iran having the leading number of cases in the world. Individuals with this disorder form clots normally, however the clots are unstable and typically degrade which results in long bleeding episodes. Most phenotypical changes in factor XIII deficiency are caused by mutations in the A subunits. | + | Factor XIII deficiency is a genetic bleeding disorder. It is an autosomal recessive disease. It is rare with Iran having the leading number of cases in the world. Individuals with this disorder form clots normally, however the clots are unstable and typically degrade which results in long bleeding episodes. Most phenotypical changes in factor XIII deficiency are caused by mutations in the A subunits<ref>https://rarediseases.org/rare-diseases/factor-xiii-deficiency/</ref>. |
The symptoms of Factor XIII deficiency vary but in 80% of cases appear after birth with a bleeding episode stemming from the umbilical stump. Bleeding can occur spontaneously or from various activities. Commonly associated symptoms include chronic nosebleeds, bleeding from the gums, discoloration of the skin, and hematomas. These individuals typically bruise easily and spontaneously. 30% of people experience spontaneous intracranial hemorrhages. In homozygous woman spontaneous recurrent miscarriages can occur. | The symptoms of Factor XIII deficiency vary but in 80% of cases appear after birth with a bleeding episode stemming from the umbilical stump. Bleeding can occur spontaneously or from various activities. Commonly associated symptoms include chronic nosebleeds, bleeding from the gums, discoloration of the skin, and hematomas. These individuals typically bruise easily and spontaneously. 30% of people experience spontaneous intracranial hemorrhages. In homozygous woman spontaneous recurrent miscarriages can occur. | ||
| Line 44: | Line 44: | ||
[[Thrombin]] | [[Thrombin]] | ||
| + | |||
| + | [https://rarediseases.org/rare-diseases/factor-xiii-deficiency/ Factor XIII Deficiency] | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 18:10, 29 April 2020
Human Coagulation Factor XIII
| |||||||||||
References
- ↑ Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016)
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016)
- ↑ https://rarediseases.org/rare-diseases/factor-xiii-deficiency/