We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:R. Jeremy Johnson/Insulin Receptor
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
===Alpha Subunits=== | ===Alpha Subunits=== | ||
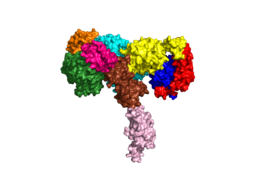
[[Image:Harrison Image2.png|thumb|right|260px|Figure 1: Insulin receptor apo receptor. Site L1' is colored a dark green, CR' is orange, L2' is bright blue, L2 is yellow, CR is red, L1 is dark blue, FnIII-1 is brown, and FnIII-2 is light pink. Insulin is shown bound and is colored dark pink. [http://www.rcsb.org/structure/6CE7 PDB 6CE7]]] | [[Image:Harrison Image2.png|thumb|right|260px|Figure 1: Insulin receptor apo receptor. Site L1' is colored a dark green, CR' is orange, L2' is bright blue, L2 is yellow, CR is red, L1 is dark blue, FnIII-1 is brown, and FnIII-2 is light pink. Insulin is shown bound and is colored dark pink. [http://www.rcsb.org/structure/6CE7 PDB 6CE7]]] | ||
| - | The alpha subunits make up the extracellular domain ([http://en.wikipedia.org/wiki/Ectodomain ectodomain]) of the insulin receptor and are the sites of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a <scene name='83/832953/Alpha_c_helix/6'>C-Terminal alpha helix</scene> (Figure 1). <ref name="Scapin"> PMID 29512653 </ref> The CT-alpha helix is unique in its position that allows it to reach across the receptor and interact with the insulin at the binding site on the opposing side of the receptor. The alpha subunits are held together by a [http://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='83/832953/Cysteine_bond/2'>cysteine residues</scene> at the CYS524 position on each alpha subunit. The disulfide bonds are important to the overall stabilization of the molecule as it binds to insulin. Two types of insulin binding sites are present in the alpha subunits, <scene name='83/832953/Sites_1_and_1_prime_location/17'>sites 1 and 1'</scene> and <scene name='83/832953/Sites_2_and_2_prime_location/13'>sites 2 and 2'</scene> (Figure 2). The sites are in pairs because of the heterodimeric nature of the receptor. Due to structural differences, as well as greater surface area and accessibility, binding sites 1 and 1' have much higher affinity than that of sites 2 and 2'. Insulin can also bind at sites 2 and 2', but the location on the back of the beta sheet of the FnIII-1 domain and lack of surface area decreases the likelihood of their binding site becoming occupied as quickly. <ref name="Uchikawa"> DOI 10.7554/eLife.48630 </ref> Cryo-EM has imaged insulin bound structures that displayed a T-shape conformation in the alpha subunits, which make up the receptors extracellular domain region.<ref name="Uchikawa" /> | + | The <scene name='83/832953/Alpha_subunits/5'>alpha subunits</scene> make up the extracellular domain ([http://en.wikipedia.org/wiki/Ectodomain ectodomain]) of the insulin receptor and are the sites of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a <scene name='83/832953/Alpha_c_helix/6'>C-Terminal alpha helix</scene> (Figure 1). <ref name="Scapin"> PMID 29512653 </ref> The CT-alpha helix is unique in its position that allows it to reach across the receptor and interact with the insulin at the binding site on the opposing side of the receptor. The alpha subunits are held together by a [http://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='83/832953/Cysteine_bond/2'>cysteine residues</scene> at the CYS524 position on each alpha subunit. The disulfide bonds are important to the overall stabilization of the molecule as it binds to insulin. Two types of insulin binding sites are present in the alpha subunits, <scene name='83/832953/Sites_1_and_1_prime_location/17'>sites 1 and 1'</scene> and <scene name='83/832953/Sites_2_and_2_prime_location/13'>sites 2 and 2'</scene> (Figure 2). The sites are in pairs because of the heterodimeric nature of the receptor. Due to structural differences, as well as greater surface area and accessibility, binding sites 1 and 1' have much higher affinity than that of sites 2 and 2'. Insulin can also bind at sites 2 and 2', but the location on the back of the beta sheet of the FnIII-1 domain and lack of surface area decreases the likelihood of their binding site becoming occupied as quickly. <ref name="Uchikawa"> DOI 10.7554/eLife.48630 </ref> Cryo-EM has imaged insulin bound structures that displayed a T-shape conformation in the alpha subunits, which make up the receptors extracellular domain region.<ref name="Uchikawa" /> |
[[Image:4 sites highlighted - Harrison.png|thumb|right|260px|Figure 2: The four binding sites of insulin. Sites 1 and 1' are colored green, sites 2 and 2' are colored red. [http://www.rcsb.org/structure/6SOF PDB 6SOF]]] | [[Image:4 sites highlighted - Harrison.png|thumb|right|260px|Figure 2: The four binding sites of insulin. Sites 1 and 1' are colored green, sites 2 and 2' are colored red. [http://www.rcsb.org/structure/6SOF PDB 6SOF]]] | ||
===Beta Subunits=== | ===Beta Subunits=== | ||
| - | The beta | + | The <scene name='83/832953/Beta_subunits/4'>beta subunits</scene> spans from the extracellular domain across the transmembrane region and into the intracellular portion of the insulin receptor. The beta subunit is composed of part of [http://en.wikipedia.org/wiki/Fibronectin fibronectin] domain III-2 and all of Fibronectin domain III-3. <ref name="Scapin" /> The beta subunit's FnIII-3 domain has links through the transmembrane region into the intracellular part of the membrane. Cryo-EM provided clear representations of the FnIII-2 and FnIII-3 domains (Figure 1) but are missing the transmembrane and intracellular regions. Although the FnIII-3 domain is connected to the transmembrane and intracellular regions, the active <scene name='83/839263/T-shape/4'>T-shape</scene> conformation (Figure 3) likely extends all the way to the tyrosine kinase domain region (see [http://www.rcsb.org/structure/4XLV PDB 4XLV]).<ref name= "Cabail"> DOI: 10.1038/ncomms7406 </ref> |
===Subunit Organization=== | ===Subunit Organization=== | ||
| Line 27: | Line 27: | ||
The structure of the extracellular domain is stabilized through multiple [http://en.wikipedia.org/wiki/Disulfide disulfide bonds]. The alpha subunits are linked through two disulfide bonds, with the main one being between <scene name='83/839263/Cys_holding_alphas_together/4'>Cys524</scene> of two adjacent alpha subuntis <ref name="Schäffer" />. <scene name='83/839263/Cys_683_holding_alphas_togethe/3'>Cys683</scene> of both alpha subunits are also held together with a disulfide bond.<ref name="Sparrow"> PMID: 9368005</ref> The alpha subunit is also attached to the beta subunit by a disulfide bond between the <scene name='83/839263/Alpha_beta_link_by_disulfide/5'>Cys647 of the alpha subunit and Cys872 of the beta subunit</scene>.<ref name="Sparrow" /> | The structure of the extracellular domain is stabilized through multiple [http://en.wikipedia.org/wiki/Disulfide disulfide bonds]. The alpha subunits are linked through two disulfide bonds, with the main one being between <scene name='83/839263/Cys_holding_alphas_together/4'>Cys524</scene> of two adjacent alpha subuntis <ref name="Schäffer" />. <scene name='83/839263/Cys_683_holding_alphas_togethe/3'>Cys683</scene> of both alpha subunits are also held together with a disulfide bond.<ref name="Sparrow"> PMID: 9368005</ref> The alpha subunit is also attached to the beta subunit by a disulfide bond between the <scene name='83/839263/Alpha_beta_link_by_disulfide/5'>Cys647 of the alpha subunit and Cys872 of the beta subunit</scene>.<ref name="Sparrow" /> | ||
| - | + | ||
== Function== | == Function== | ||
| Line 34: | Line 34: | ||
The insulin molecules bind to these sites mostly through [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic interactions], with some of the most crucial residues at sites 1 and 1' being between <scene name='83/839263/Residues_of_site_1_binding/8'>Cys A7, Cys B7, and His B5 of insulin and Pro495, Phe497, and Arg498</scene> of the insulin receptor FnIII-1 domain <ref name="Uchikawa" />. Despite some of the residues included being charged they can still interact hydrophobically in this binding site. For example, due to arginine carrying its positive charge at the end of the side chain, <scene name='83/839263/Arginine_bending/1'> the side chain is bent</scene> to allow the hydrophobic part of the side chain to interact with the other hydrophobic residues. The alpha subunits also have significant <scene name='83/832953/Cysteine_bond/3'>disulfide linkages</scene> that help maintain a compact binging site. At sites 2 and 2', the major residues contributing to these hydrophobic interactions are the <scene name='83/839263/Site_2_residues_hydrophobic/4'>Leu 486, Leu 552, and Pro537 of the insulin receptor and Leu A13, Try A14, Leu A16, Leu B6, Ala B14, Leu B17 and Val B18 of the insulin molecule</scene><ref name="Uchikawa" />. While the majority of the binding interactions appear similar, sites 1 and 1' have a higher binding affinity than sites 2 and 2' due to site 1 having a larger surface area (706 Å<sup>2</sup>) exposed for insulin to bind to compared to site 2 (394 Å<sup>2</sup>)<ref name="Uchikawa" />. The binding interactions of the insulin molecules in sites 1 and 1' are facilitated by hydrophobic residues of an <scene name='83/839263/Insulin_bound_to_site_1/4'>alpha-helix</scene> of the insulin receptor. The insulin molecules in sites 2 and 2' primarily interact with the residues that comprise some of the <scene name='83/839263/Insulin_in_site_2_with_beta_sh/7'>beta-sheets</scene> of the insulin receptor. | The insulin molecules bind to these sites mostly through [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic interactions], with some of the most crucial residues at sites 1 and 1' being between <scene name='83/839263/Residues_of_site_1_binding/8'>Cys A7, Cys B7, and His B5 of insulin and Pro495, Phe497, and Arg498</scene> of the insulin receptor FnIII-1 domain <ref name="Uchikawa" />. Despite some of the residues included being charged they can still interact hydrophobically in this binding site. For example, due to arginine carrying its positive charge at the end of the side chain, <scene name='83/839263/Arginine_bending/1'> the side chain is bent</scene> to allow the hydrophobic part of the side chain to interact with the other hydrophobic residues. The alpha subunits also have significant <scene name='83/832953/Cysteine_bond/3'>disulfide linkages</scene> that help maintain a compact binging site. At sites 2 and 2', the major residues contributing to these hydrophobic interactions are the <scene name='83/839263/Site_2_residues_hydrophobic/4'>Leu 486, Leu 552, and Pro537 of the insulin receptor and Leu A13, Try A14, Leu A16, Leu B6, Ala B14, Leu B17 and Val B18 of the insulin molecule</scene><ref name="Uchikawa" />. While the majority of the binding interactions appear similar, sites 1 and 1' have a higher binding affinity than sites 2 and 2' due to site 1 having a larger surface area (706 Å<sup>2</sup>) exposed for insulin to bind to compared to site 2 (394 Å<sup>2</sup>)<ref name="Uchikawa" />. The binding interactions of the insulin molecules in sites 1 and 1' are facilitated by hydrophobic residues of an <scene name='83/839263/Insulin_bound_to_site_1/4'>alpha-helix</scene> of the insulin receptor. The insulin molecules in sites 2 and 2' primarily interact with the residues that comprise some of the <scene name='83/839263/Insulin_in_site_2_with_beta_sh/7'>beta-sheets</scene> of the insulin receptor. | ||
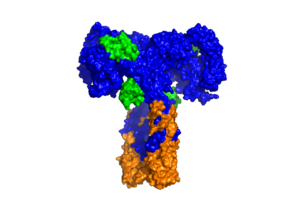
| - | + | [[Image:SurfaceIR.png|thumb|right|300px|Figure 3: Surface representation of the insulin receptor in the active "T" shape conformation with four insulins bound (green). [http://www.rcsb.org/structure/6SOF PDB 6SOF]]] | |
At <scene name='83/832953/Sites_1_and_1_prime_location/17'>binding sites 1 and 1'</scene>, a <scene name='83/832953/Tripartite_interaction/8'>tripartite interaction</scene> occurs between three critical parts of the alpha subunits of the insulin receptor. <ref name="Uchikawa" /> The entire interface of the tripartite interaction involves many residues that are involved with intra-protomer ionic and hydrogen bonding at the binding site. The α-CT chain and the FnIII-1 domain region become in close proximity during the conformational change of the insulin receptor and their interaction involves the following residues: <scene name='83/832953/Alpha_ct_and_fniii-1/7'>ASP496, ARG498, and ASP499 on the FnIII-1 domain</scene> and the <scene name='83/832953/Alpha_ct_and_fniii-1/9'>LYS703, GLU706, and ASP707 on the α-CT domain</scene>. This duo then interacts with the L1 region, specifically ARG14, creating an ideal <scene name='83/832953/Tripartite_interaction/9'>binding site</scene> for the insulin ligand. The FnIII-1 and α-CT are interacting from the two different alpha subunits, which displays a "cross linking" scenario where the domains of the heterodimer can intertwine with each other. The tripartite interaction between the α-CT chain, FnIII-1 domain, and the L1 region is important because it allows for a strong interaction between two subunits of the insulin receptor that maintains and stabilizes the T-shape activation state for the rest of the downstream signaling to occur. <ref name="Uchikawa" /> | At <scene name='83/832953/Sites_1_and_1_prime_location/17'>binding sites 1 and 1'</scene>, a <scene name='83/832953/Tripartite_interaction/8'>tripartite interaction</scene> occurs between three critical parts of the alpha subunits of the insulin receptor. <ref name="Uchikawa" /> The entire interface of the tripartite interaction involves many residues that are involved with intra-protomer ionic and hydrogen bonding at the binding site. The α-CT chain and the FnIII-1 domain region become in close proximity during the conformational change of the insulin receptor and their interaction involves the following residues: <scene name='83/832953/Alpha_ct_and_fniii-1/7'>ASP496, ARG498, and ASP499 on the FnIII-1 domain</scene> and the <scene name='83/832953/Alpha_ct_and_fniii-1/9'>LYS703, GLU706, and ASP707 on the α-CT domain</scene>. This duo then interacts with the L1 region, specifically ARG14, creating an ideal <scene name='83/832953/Tripartite_interaction/9'>binding site</scene> for the insulin ligand. The FnIII-1 and α-CT are interacting from the two different alpha subunits, which displays a "cross linking" scenario where the domains of the heterodimer can intertwine with each other. The tripartite interaction between the α-CT chain, FnIII-1 domain, and the L1 region is important because it allows for a strong interaction between two subunits of the insulin receptor that maintains and stabilizes the T-shape activation state for the rest of the downstream signaling to occur. <ref name="Uchikawa" /> | ||
| Line 40: | Line 40: | ||
===Conformational Changes=== | ===Conformational Changes=== | ||
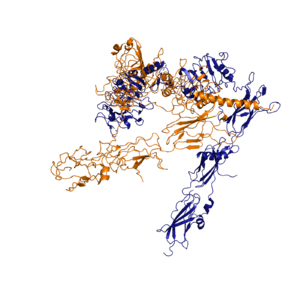
| - | [[Image:image 6.png|thumb|left|250px|Figure 3: Conformational change of insulin receptor protomer from inactive (blue) to active (orange) form upon insulin binding. Inactive state PDB: 4zxb. Active state PDB: 6sof]] | ||
The conformational change between the inverted, inactive <scene name='83/839263/V_shape/3'>"V" shape</scene> and the active <scene name='83/839263/T-shape/4'>"T" shape</scene> of the insulin receptor is induced by insulin binding. The T shape conformation is well observed in the alpha subunit. It is horizontally composed of L1, CR (including the <scene name='83/832953/Alpha_c_helix/9'>α-CT chain</scene>), and L2 domains and vertically composed of the FnIII-1, 2, and 3 domains (Figure 1). The proper conformational change of the ectodomain of the insulin receptor is crucial for transmitting the signal into the cell. The movements extracellularly cause the two receptor tyrosine kinase domains intracellularly to become close enough to each other to [http://en.wikipedia.org/wiki/Autophosphorylation autophosphorylate].<ref name="Boucher" /> This autophosphorylation leads enzymes to become activated in the cell that carries out processes related to insulin signaling such as metabolism and growth. <ref name="Boucher" /> | The conformational change between the inverted, inactive <scene name='83/839263/V_shape/3'>"V" shape</scene> and the active <scene name='83/839263/T-shape/4'>"T" shape</scene> of the insulin receptor is induced by insulin binding. The T shape conformation is well observed in the alpha subunit. It is horizontally composed of L1, CR (including the <scene name='83/832953/Alpha_c_helix/9'>α-CT chain</scene>), and L2 domains and vertically composed of the FnIII-1, 2, and 3 domains (Figure 1). The proper conformational change of the ectodomain of the insulin receptor is crucial for transmitting the signal into the cell. The movements extracellularly cause the two receptor tyrosine kinase domains intracellularly to become close enough to each other to [http://en.wikipedia.org/wiki/Autophosphorylation autophosphorylate].<ref name="Boucher" /> This autophosphorylation leads enzymes to become activated in the cell that carries out processes related to insulin signaling such as metabolism and growth. <ref name="Boucher" /> | ||
| - | + | [[Image:image 6.png|thumb|right|300px|Figure 4: Conformational change of insulin receptor protomer from inactive (blue) to active (orange) form upon insulin binding. [http://www.rcsb.org/structure/4ZXB Inactive PDB 4ZXB] [http://www.rcsb.org/structure/6SOF Active PDB 6SOF]]] | |
When an insulin molecule binds to site 1 of the alpha subunit, the respective protomer is recruited and a slight inward movement of the <scene name='83/839263/Fniii_domains/1'>Fibronectin type III domains</scene> of the beta subunit is initiated. This is accomplished by the formation of several [http://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridges], specifically between <scene name='83/839263/Salt_bridges/1'>Arg498 and Asp499 of the FnIII-1 and Lys703, Glu706, and Asp707 of the alpha-CT</scene> <ref name="Uchikawa" />. Binding of insulin to both protomers establishes a full activation of the insulin receptor. This activation is demonstrated through the inward movement of both protomers. This motion has been referred to as a "hinge" motion <ref name="Uchikawa" /> as both protomers "swing" in towards one another. Figure 3 depicts the conformational change and "hinge motion" between the inactive and active forms of an insulin receptor protomer. Upon insulin binding, the beta subunits of the inactive form, shown in blue, are "swung" inward to the active form, shown in orange. When the receptor is in an <scene name='83/832953/Inactive_insulin_receptor/6'>inverted V shape</scene>, the FnIII-3 domains are separated by about 120Å. <ref name= "Mckern"> PMID: 16957736</ref> This distance prevents the initiation of autophosphorylation and downstream signaling by the tyrosine kinase domains on the intracellular side of the receptor. Upon the binding of insulin to multiple binding sites, the conformation change will begin and bring the FnIII-3 domains within 40Å of each other to induce the <scene name='83/832953/Ir_dimer_t_state/4'>T shape</scene> conformation. <ref> DOI 10.1038/s41467-018-06826-6</ref> <ref name="Uchikawa" /> As the fibronectin type III domains of the beta subunit swing inward, the alpha subunits also undergo a conformational change upon insulin binding. As insulin binds to site 1, the leucine-rich region of one protomer interacts with the ''alpha''-CT and the FNIII-1 domains of the other protomer to form the <scene name='83/839263/Tripartite_interface/2'>tripartite interface</scene> binding site.<ref name="Uchikawa" /> For the tripartite interface to form, the alpha subunits of each protomer must undergo a "folding" motion. While there is an explanation for which conformational changes of the insulin receptor take place, there is no full explanation for the exact mechanism by which the conformational changes are executed in the receptor.<ref name="Uchikawa" /> | When an insulin molecule binds to site 1 of the alpha subunit, the respective protomer is recruited and a slight inward movement of the <scene name='83/839263/Fniii_domains/1'>Fibronectin type III domains</scene> of the beta subunit is initiated. This is accomplished by the formation of several [http://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridges], specifically between <scene name='83/839263/Salt_bridges/1'>Arg498 and Asp499 of the FnIII-1 and Lys703, Glu706, and Asp707 of the alpha-CT</scene> <ref name="Uchikawa" />. Binding of insulin to both protomers establishes a full activation of the insulin receptor. This activation is demonstrated through the inward movement of both protomers. This motion has been referred to as a "hinge" motion <ref name="Uchikawa" /> as both protomers "swing" in towards one another. Figure 3 depicts the conformational change and "hinge motion" between the inactive and active forms of an insulin receptor protomer. Upon insulin binding, the beta subunits of the inactive form, shown in blue, are "swung" inward to the active form, shown in orange. When the receptor is in an <scene name='83/832953/Inactive_insulin_receptor/6'>inverted V shape</scene>, the FnIII-3 domains are separated by about 120Å. <ref name= "Mckern"> PMID: 16957736</ref> This distance prevents the initiation of autophosphorylation and downstream signaling by the tyrosine kinase domains on the intracellular side of the receptor. Upon the binding of insulin to multiple binding sites, the conformation change will begin and bring the FnIII-3 domains within 40Å of each other to induce the <scene name='83/832953/Ir_dimer_t_state/4'>T shape</scene> conformation. <ref> DOI 10.1038/s41467-018-06826-6</ref> <ref name="Uchikawa" /> As the fibronectin type III domains of the beta subunit swing inward, the alpha subunits also undergo a conformational change upon insulin binding. As insulin binds to site 1, the leucine-rich region of one protomer interacts with the ''alpha''-CT and the FNIII-1 domains of the other protomer to form the <scene name='83/839263/Tripartite_interface/2'>tripartite interface</scene> binding site.<ref name="Uchikawa" /> For the tripartite interface to form, the alpha subunits of each protomer must undergo a "folding" motion. While there is an explanation for which conformational changes of the insulin receptor take place, there is no full explanation for the exact mechanism by which the conformational changes are executed in the receptor.<ref name="Uchikawa" /> | ||
Revision as of 16:15, 8 May 2020
Insulin Receptor
| |||||||||||
References
- ↑ 1.0 1.1 1.2 De Meyts P. The structural basis of insulin and insulin-like growth factor-I receptor binding and negative co-operativity, and its relevance to mitogenic versus metabolic signalling. Diabetologia. 1994 Sep;37 Suppl 2:S135-48. doi: 10.1007/bf00400837. PMID:7821729 doi:http://dx.doi.org/10.1007/bf00400837
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1). pii: 6/1/a009191. doi:, 10.1101/cshperspect.a009191. PMID:24384568 doi:http://dx.doi.org/10.1101/cshperspect.a009191
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ 4.0 4.1 4.2 Scapin G, Dandey VP, Zhang Z, Prosise W, Hruza A, Kelly T, Mayhood T, Strickland C, Potter CS, Carragher B. Structure of the Insulin Receptor-Insulin Complex by Single Particle CryoEM analysis. Nature. 2018 Feb 28. pii: nature26153. doi: 10.1038/nature26153. PMID:29512653 doi:http://dx.doi.org/10.1038/nature26153
- ↑ 5.0 5.1 Schaffer L, Ljungqvist L. Identification of a disulfide bridge connecting the alpha-subunits of the extracellular domain of the insulin receptor. Biochem Biophys Res Commun. 1992 Dec 15;189(2):650-3. PMID:1472036
- ↑ White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1-4. PMID:8276779
- ↑ 7.0 7.1 7.2 Tatulian SA. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub , 2015 Sep 3. PMID:26322622 doi:http://dx.doi.org/10.1021/acs.biochem.5b00805
- ↑ Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997 Sep 15;16(18):5572-81. PMID:9312016 doi:10.1093/emboj/16.18.5572
- ↑ Cabail MZ, Li S, Lemmon E, Bowen ME, Hubbard SR, Miller WT. The insulin and IGF1 receptor kinase domains are functional dimers in the activated state. Nat Commun. 2015 Mar 11;6:6406. doi: 10.1038/ncomms7406. PMID:25758790 doi:http://dx.doi.org/10.1038/ncomms7406
- ↑ 10.0 10.1 Sparrow LG, McKern NM, Gorman JJ, Strike PM, Robinson CP, Bentley JD, Ward CW. The disulfide bonds in the C-terminal domains of the human insulin receptor ectodomain. J Biol Chem. 1997 Nov 21;272(47):29460-7. doi: 10.1074/jbc.272.47.29460. PMID:9368005 doi:http://dx.doi.org/10.1074/jbc.272.47.29460
- ↑ McKern NM, Lawrence MC, Streltsov VA, Lou MZ, Adams TE, Lovrecz GO, Elleman TC, Richards KM, Bentley JD, Pilling PA, Hoyne PA, Cartledge KA, Pham TM, Lewis JL, Sankovich SE, Stoichevska V, Da Silva E, Robinson CP, Frenkel MJ, Sparrow LG, Fernley RT, Epa VC, Ward CW. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature. 2006 Sep 14;443(7108):218-21. Epub 2006 Sep 6. PMID:16957736 doi:10.1038/nature05106
- ↑ Weis F, Menting JG, Margetts MB, Chan SJ, Xu Y, Tennagels N, Wohlfart P, Langer T, Muller CW, Dreyer MK, Lawrence MC. The signalling conformation of the insulin receptor ectodomain. Nat Commun. 2018 Oct 24;9(1):4420. doi: 10.1038/s41467-018-06826-6. PMID:30356040 doi:http://dx.doi.org/10.1038/s41467-018-06826-6
- ↑ 13.0 13.1 13.2 13.3 Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science. 2016 Oct 7;354(6308):69-73. doi: 10.1126/science.aaf5094. PMID:27846494 doi:http://dx.doi.org/10.1126/science.aaf5094
Student Contributors
Abby Hillan
Alyssa Ritter
Andrew Scheel
Harrison Smith
Maxwell Todd