User:Tania Girao Mangolini/Sandbox 1
From Proteopedia
| Line 18: | Line 18: | ||
The following image shows the reactions related to the HRP C1A's Peroxydase Cycle: | The following image shows the reactions related to the HRP C1A's Peroxydase Cycle: | ||
| - | [[Image:Horseadish_peroxydase_cycle | + | [[Image:Horseadish_peroxydase_cycle.png|300px]] |
Revision as of 02:46, 1 June 2020
HORSERADISH PEROXIDASE C1A
|
Horseadish (Armoracia rusticana) is a plant that belongs to the Brassicaceae family. The roots of this plant are rich in peroxidases, being the HRP C isozymes the most common ones. [1] However, most of the HRP research has focused on one isozyme: (1H58) [2].
Structural highlights
HRP C1A is composed by 308 residues and the residue at is Ile according to the GenBank entry M37156.1 but Tyr according to the GenBank entry HE963800.1 [2].
The molecule has a predominantly α-helical , with the exception of one short β-sheet region, and it is separated into a distal and a proximal region, each one with a .
In the center of HRP C1A there is a , which is linked to the molecule by a coordinate bond of the heme iron with a conserved residue [1].
There are sites for N glycosylation in the loop regions, at residues[1]. All these glycosylated Asn residues are located on the surface of C1A[2].
Other residues play essencial roles in the the molecule, as the and , which are related to the formation and stabilization of (1HCH), that is the active form of this enzyme.
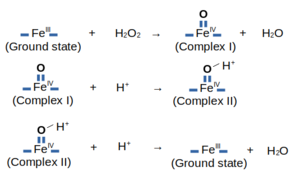
The following image shows the reactions related to the HRP C1A's Peroxydase Cycle:
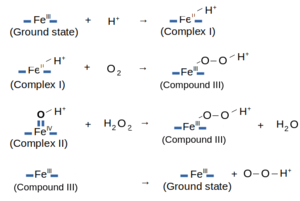
The following image shows the reactions related to the HRP C1A's Oxidase Cycle:
(1H55)
(1H57)
References
- ↑ 1.0 1.1 1.2 Veitch, N.C. Horseadich peroxidase: a modern view of a classic enzyme 65:249-259 (2004). DOI: 10.1016/j.phytochem.2003.10.022
- ↑ 2.0 2.1 2.2 Krainer, F.W; GLIEDER, A. An updated view on horseradish peroxidases: recombinant production and biotechnological applications v. 99, pages 1611–1625 (2015). DOI: 10.1007/s00253-014-6346-7