We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Andre Wu Le Chun/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structural highlights == | == Structural highlights == | ||
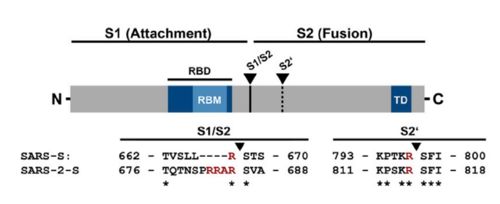
| - | <scene name='84/844931/6vsb/2'>6vsb</scene> is a homotrimer transmembrane glycoprotein(S) protruding from the viral surface¹. It is composed by two subunits that are fundamental for the virus entry in the cell: The S1 subunit, which contains the receptor binding domain (<scene name='84/844931/S_receptor-binding_domain/1'>RBD</scene>), is responsible for binding to the host cell receptor, and the S2 subunit, responsible for fusion of the viral and cellular membranes. In order to allow the binding of the RBD in S1 with the host cell, the spike protein goes through conformational changes that | + | <scene name='84/844931/6vsb/2'>6vsb</scene> is a homotrimer transmembrane glycoprotein(S) protruding from the viral surface¹. It is composed by two subunits that are fundamental for the virus entry in the cell: The S1 subunit, which contains the receptor binding domain (<scene name='84/844931/S_receptor-binding_domain/1'>RBD</scene>), is responsible for binding to the host cell receptor, and the S2 subunit, responsible for fusion of the viral and cellular membranes. In order to allow the binding of the RBD in S1 with the host cell, the spike protein goes through conformational changes that make the binding domain assume a up conformation, thus, exposing the spike protein to its target receptor. |
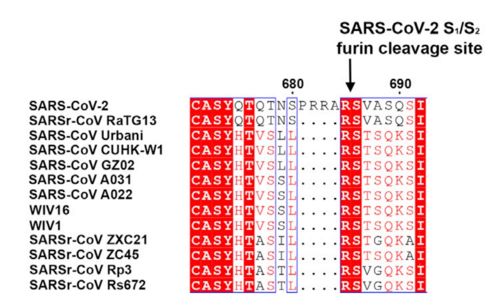
| - | This protein also shares amino acid sequence identity of 76% with SARS-CoV, however, as shown in the figure bellow, it has inserted amino acids that indicate a furin like cleavage site in the S1/S2 boundary. that must be primed in order to enable the viral pathogenicity. | + | This protein also shares amino acid sequence identity of 76% with SARS-CoV, however, as shown in the figure bellow, it has inserted amino acids that indicate a furin like cleavage site in the S1/S2 boundary. that must be primed in order to enable the viral pathogenicity. Regardind its secundary structure, units of beta-sheets, alpha-helices and loops can be observed thoughout the protein. |
| Line 27: | Line 27: | ||
== Relevance == | == Relevance == | ||
| - | Due to its role in the infection process, the spike glyprotein may be a potential target of studies that seek methods of preventing | + | Due to its role in the infection process, the spike glyprotein may be a potential target of studies that seek methods of preventing COVID-19. These include the development of vaccines based on antibodies that are able to block protein's structure of the protein and on it's recognition/biding mechanisms. For instance, avoiding the cleavage of the furin, located on the B domain of the protein, by the host's proteases could be a way of viral inhibition. Comprehending how the protein can be bound to antibodies could also help the scientific community to prepare for future SARS coronavirus outbreaks that may happen in the future. |
== Interaction with angiotensin-converting enzime 2 == | == Interaction with angiotensin-converting enzime 2 == | ||
The interaction between the 2019-nCov and the host cell begins with the recognition of the ACE2, a dimeric protein. Then, the S1 subunit moves, modifying the protein's conformation in way that receptor-binding domain becomes available to connect itself to the peptidase domain of target cell. Due to the existence of four amino acids in between the S1 and S2, a furin cleavage site is featured in the protein. The cleavage of this site has potential relevance as it could lead to later priming at the S2' site by a serine protease, TMPRSS2. Once attached on the surface of ACE2, the cleavage of S2' happens and the fusion mechanism is activated. Then, the protein goes through conformational changes, the protein structure assumes a conformation which is suitable for binding with the ACE2 receptor. At that instance, the spike protein is found in a "up" conformation, hence the protein's name. It binds itself to the ACE2 with high affinity (15nM). In order to activate infection, the spike protein is cleaved by TMPRSS2, a host's serine protease, also fundamental for the viral infection. Furine cleavage site | The interaction between the 2019-nCov and the host cell begins with the recognition of the ACE2, a dimeric protein. Then, the S1 subunit moves, modifying the protein's conformation in way that receptor-binding domain becomes available to connect itself to the peptidase domain of target cell. Due to the existence of four amino acids in between the S1 and S2, a furin cleavage site is featured in the protein. The cleavage of this site has potential relevance as it could lead to later priming at the S2' site by a serine protease, TMPRSS2. Once attached on the surface of ACE2, the cleavage of S2' happens and the fusion mechanism is activated. Then, the protein goes through conformational changes, the protein structure assumes a conformation which is suitable for binding with the ACE2 receptor. At that instance, the spike protein is found in a "up" conformation, hence the protein's name. It binds itself to the ACE2 with high affinity (15nM). In order to activate infection, the spike protein is cleaved by TMPRSS2, a host's serine protease, also fundamental for the viral infection. Furine cleavage site | ||
| + | |||
[[Image:Spike.jpg|500px|]] | [[Image:Spike.jpg|500px|]] | ||
Figure 2: Alignment between S protein of SARS and SARS-2 (Hoffmann et al., 2020). | Figure 2: Alignment between S protein of SARS and SARS-2 (Hoffmann et al., 2020). | ||
Revision as of 20:56, 21 June 2020
6vsb
Prefusion 2019-nCoV spike glycoprotein with a single receptor-binding domain up
| |||||||||||
References
¹https://www.sciencedirect.com/science/article/pii/S0065352719300284 ²https://www.biorxiv.org/content/10.1101/2020.02.19.956581v1.full https://www.sciencedirect.com/science/article/pii/S0092867420302294
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644