User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
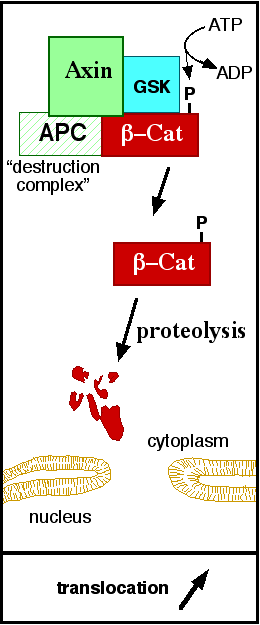
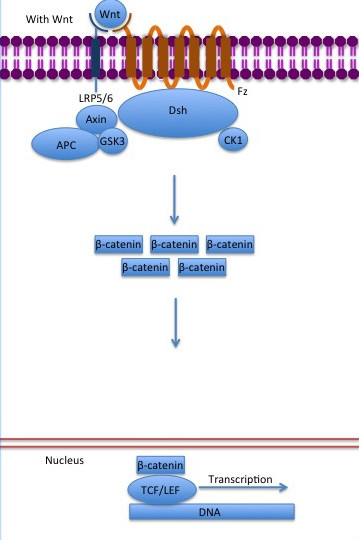
The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 3) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> | The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 3) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> | ||
| - | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT | + | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT</scene> (here ICAT is represented in orange and is a full length structure from ''Homo sapiens''; ß-catenin is shown in green and is from ''Mus musculus''). ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> |
This interaction can be divided in two regions: the <scene name='84/848919/Extendedregionicat_bcat_5_10/2'>ICAT extended C-terminal region bind to the ß-catenin ARM 5-10 </scene> and the <scene name='84/848919/Helicalicatdomain_bcat11_12/4'>ICAT helical N-terminal domain interacts with the ARM repeat 11 and 12</scene>. The first one overlaps with others ß-catenin ligands and is known for its several <scene name='84/848919/Hydrophobic_icat_bcat/1'>hydrophobic interactions</scene> (for example, Val68, Met69, and Phe71 interact with hydrophobic sites on the surface of ß-catenin) and <scene name='84/848919/2saltbridge_icat_bcat/1'> two salt bridges </scene> - Asp66 and Glu75 form salt bridges with ß-catenin residues Lys435 (repeat 8) and Lys312 (repeat 5). There are other polar contacts to stabilize the protein-protein binding. Finally, the interaction between the ICAT helical domain and the two last ARM repeat is water-mediated contact (with ARM 11) and hydrophobic interactions (ARM 12). The hydrophobic interactions are stabilized by the connections between the aliphatic portion of the <scene name='84/848919/Hydrophbic_lys19_icat_bcat/1'>Lys19 side chain and the aromatic rings of Phe660 (ARM 12) and Phe40 (ICAT)</scene>. Lys19 also forms a <scene name='84/848919/Salt_bridge_lys19_icat_bcat/1'>salt bridge</scene> with ß-catenin Glu664 (repeat 12). Another polar interaction occurs between <scene name='84/848919/Arg_glu_icat_bcat/1'>ICAT Glu37 and ß-catenin Arg661</scene>. <ref name=Daniels & Weis 2002> Daniels, D. L., & Weis, W. I. (2002). ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Molecular cell, 10(3), 573–584. https://doi.org/10.1016/s1097-2765(02)00631-7. </ref> | This interaction can be divided in two regions: the <scene name='84/848919/Extendedregionicat_bcat_5_10/2'>ICAT extended C-terminal region bind to the ß-catenin ARM 5-10 </scene> and the <scene name='84/848919/Helicalicatdomain_bcat11_12/4'>ICAT helical N-terminal domain interacts with the ARM repeat 11 and 12</scene>. The first one overlaps with others ß-catenin ligands and is known for its several <scene name='84/848919/Hydrophobic_icat_bcat/1'>hydrophobic interactions</scene> (for example, Val68, Met69, and Phe71 interact with hydrophobic sites on the surface of ß-catenin) and <scene name='84/848919/2saltbridge_icat_bcat/1'> two salt bridges </scene> - Asp66 and Glu75 form salt bridges with ß-catenin residues Lys435 (repeat 8) and Lys312 (repeat 5). There are other polar contacts to stabilize the protein-protein binding. Finally, the interaction between the ICAT helical domain and the two last ARM repeat is water-mediated contact (with ARM 11) and hydrophobic interactions (ARM 12). The hydrophobic interactions are stabilized by the connections between the aliphatic portion of the <scene name='84/848919/Hydrophbic_lys19_icat_bcat/1'>Lys19 side chain and the aromatic rings of Phe660 (ARM 12) and Phe40 (ICAT)</scene>. Lys19 also forms a <scene name='84/848919/Salt_bridge_lys19_icat_bcat/1'>salt bridge</scene> with ß-catenin Glu664 (repeat 12). Another polar interaction occurs between <scene name='84/848919/Arg_glu_icat_bcat/1'>ICAT Glu37 and ß-catenin Arg661</scene>. <ref name=Daniels & Weis 2002> Daniels, D. L., & Weis, W. I. (2002). ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Molecular cell, 10(3), 573–584. https://doi.org/10.1016/s1097-2765(02)00631-7. </ref> | ||
Revision as of 18:20, 9 July 2020
ß-catenin
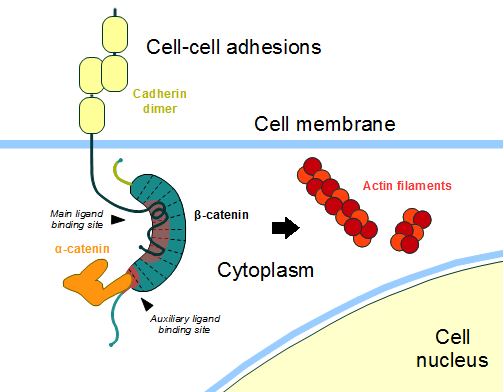
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||