User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==Structure== | ==Structure== | ||
| - | The zebrafish ([https://pt.wikipedia.org/wiki/Danio_rerio ''Danio rerio'']) <scene name='84/848919/Betacateninacoloridaartigo/2'>ß-catenin</scene> ([http://www.rcsb.org/structure/2Z6G 2Z6G]) contains residues 126-681 and a central core of <scene name='84/848919/Armrepeatsdomain/1'>12 armadillo repeats domain</scene> and an alpha helix, the <scene name='84/848919/C-helix3correta/1'>helix-C</scene>, at the | + | The zebrafish ([https://pt.wikipedia.org/wiki/Danio_rerio ''Danio rerio'']) <scene name='84/848919/Betacateninacoloridaartigo/2'>ß-catenin</scene> ([http://www.rcsb.org/structure/2Z6G 2Z6G]) contains residues 126-681 and a central core of <scene name='84/848919/Armrepeatsdomain/1'>12 armadillo repeats domain</scene> and an alpha helix, the <scene name='84/848919/C-helix3correta/1'>helix-C</scene>, at the ß-catenin C-terminal domain. |
| - | + | The terminal domains sequences mediate some of the protein interactions and are negatively charged. It is observed that the <scene name='84/848919/C-helix3correta/1'>helix-C constitutes the C-terminal domain</scene>. The N terminus of the first armadillo repeat has an <scene name='84/848919/Correton-terminushelix/1'>extra alpha helix</scene> and its helix 1 and 2 fuse into one extended and twisted helix. Both N- and C-terminal domains do not interact with the armadillo repeat domain. <ref name="xing2009" /> | |
| - | + | The armadillo domain is more conserved than the terminal domains. It is made of 12 armadillo repeats each one with <scene name='84/848919/Arm_3_helices/1'>three alpha helices connected by loops </scene> (as shown in ARM repeat 2), except for the <scene name='84/848919/Arm_repeat_7/1'>ARM repeat 7, which has two helices</scene>. Furthermore, it has a particular site which is positively charged, constituting the <scene name='84/848919/Armbidingsurface/1'>binding surface</scene> for the majority of ß-catenin ligands. Because the armadillo domain is positively while the terminal tails are negatively charged, their interactions are nonspecific. It is proposed that both tails act like chaperones - they might avoid nonspecific protein interactions of the ARM repeat domain and its self-aggregation.<ref name="xing2009" /> | |
| - | + | ||
In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged. The C-helix caps the {{Template:ColorKey_Hydrophobic}} <scene name='84/848919/Hydrophilichelixc/1'>surface formed by the C-terminal end of the last armadillo repeats.</scene>. However, the other side of the surface, exposed to solvent, is composed of {{Template:ColorKey_Polar}} residues. Thereby, this structure forms part of the superhelical structure core of ß-catenin together with armadillo repeat domain. <ref name="xing2009" /> | In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged. The C-helix caps the {{Template:ColorKey_Hydrophobic}} <scene name='84/848919/Hydrophilichelixc/1'>surface formed by the C-terminal end of the last armadillo repeats.</scene>. However, the other side of the surface, exposed to solvent, is composed of {{Template:ColorKey_Polar}} residues. Thereby, this structure forms part of the superhelical structure core of ß-catenin together with armadillo repeat domain. <ref name="xing2009" /> | ||
It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> | It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> | ||
Revision as of 22:12, 31 July 2020
ß-catenin
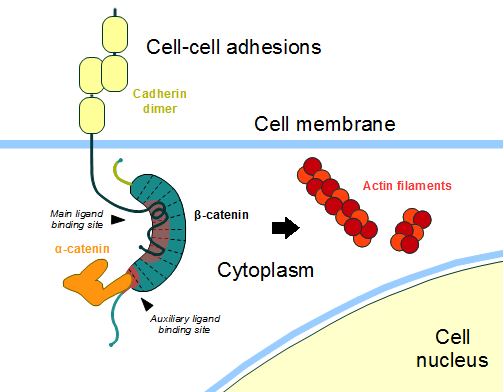
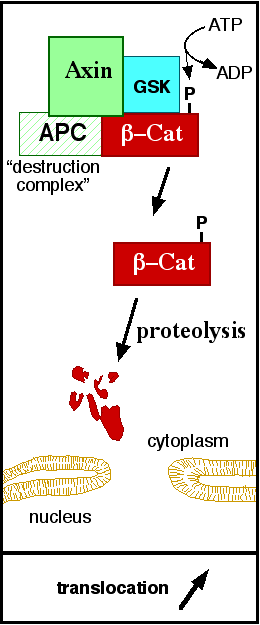
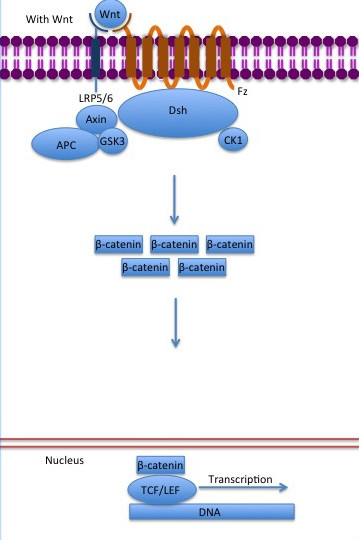
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||