Farnesyl diphosphate synthase
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | ||
Revision as of 14:38, 14 July 2020
Contents |
Function
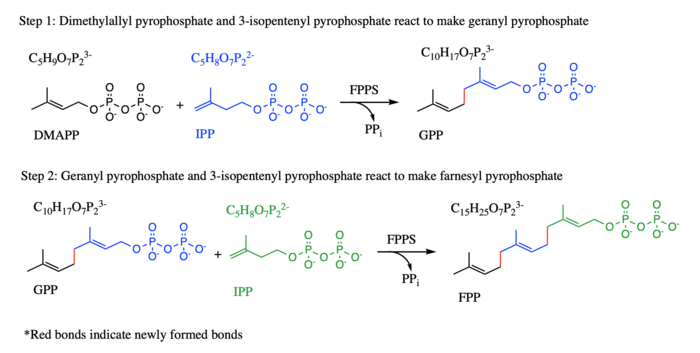
Farnesyl pyrophospate synthase (FPPS), also named Farnesyl diphosphate synthase (FPS), is a chain elongation enzyme that catalyzes carbon-carbon formation in two consecutive condensation reactions that convert one equivalent of dimethylallyl diphosphate (DMAPP) and two equivalents of isopentyl diphosphates (IPP) into one equivalent of Farnesyl pyrophospate. (FPP) [1] In the first step, IPP and its isomer DMAPP react to form a ten-carbon geranyl diphosphate (GPP), while in the second step, the product geranyl diphosphate from the first step and an additional IPP molecule react to make FPP (see diagram). It is an essential enzyme in the biosynthesis of mevalonate, isoprenoids, and sterols in a variety of organisms. FFPS has been studied in conjunction with different parasites. TcFPPS refers to FPPS in the Typanosoma cruzi parasite while LmFPPS refers to FPPS in the Leishmania major parasite. Bisphosphonates have been shown to inhibit FPPS and are currently being used as antiparasitic drugs as well as a treatment for various bone diseases.
Relevance
FPS bisphosphonate inhibitors (like zoledronic acid) are used as drugs for treatment of bone resorption diseases[2]. FPS inhibitor Alendronate or Fosamax is used in treatment of osteoporosis[3]. Bisphosphonates have been explored as antiparasitic drugs with FPPS because current treatments are expensive, have low efficacy, and have dangerous side effects. FPPS is important in the lengthening of hydrophobic chains and determination of their specificity.
Structure
FPPS exists as a homodimer, with each monomer having an active site. The monomers have the characteristic FPPS fold of a ten-helix bundle and four other helices that run perpendicular to the bundle. There are two substrate sites, one is allylic and the other is homoallylic. GPP and DMAPP bind to the allylic site, while IPP binds to the homoallylic site. These two sites are connected to the top of the bundle and exist as part of a cavity. [1] Another characteristic feature of all FPPS enzymes are two highly conserved aspartate rich motifs. These motifs are called First Aspartate Rich Motif (FARM) and Second Aspartate Rich Motif (SARM), and have sequences of DDXX(XX)D and DDXXD respectively. FARM and SARM are found on opposite sides on the active site cavity facing one another. [2]
When FPPS interacts with bisphosphonates, the bisphosphonates bind in the homoallylic binding sites and are coordinated by three divalent cations (Ca 2+ or Mg 2+). FPS contains and binds ; see also , (PDB code 2opm)[4], water molecules are shown as red spheres.
3D structures of farnesyl diphosphate synthase
Farnesyl diphosphate synthase 3D structures
</StructureSection>
|
References
- ↑ Schulbach MC, Mahapatra S, Macchia M, Barontini S, Papi C, Minutolo F, Bertini S, Brennan PJ, Crick DC. Purification, enzymatic characterization, and inhibition of the Z-farnesyl diphosphate synthase from Mycobacterium tuberculosis. J Biol Chem. 2001 Apr 13;276(15):11624-30. Epub 2001 Jan 4. PMID:11152452 doi:http://dx.doi.org/10.1074/jbc.M007168200
- ↑ Das S, Edwards PA, Crockett JC, Rogers MJ. Upregulation of endogenous farnesyl diphosphate synthase overcomes the inhibitory effect of bisphosphonate on protein prenylation in Hela cells. Biochim Biophys Acta. 2014 Apr 4;1841(4):569-73. doi:, 10.1016/j.bbalip.2013.12.010. Epub 2013 Dec 22. PMID:24369118 doi:http://dx.doi.org/10.1016/j.bbalip.2013.12.010
- ↑ Selby P. Alendronate treatment for osteoporosis: a review of the clinical evidence. Osteoporos Int. 1996;6(6):419-26. doi: 10.1007/bf01629572. PMID:9116385 doi:http://dx.doi.org/10.1007/bf01629572
- ↑ Zhang Y, Cao R, Yin F, Hudock MP, Guo RT, Krysiak K, Mukherjee S, Gao YG, Robinson H, Song Y, No JH, Bergan K, Leon A, Cass L, Goddard A, Chang TK, Lin FY, Van Beek E, Papapoulos S, Wang AH, Kubo T, Ochi M, Mukkamala D, Oldfield E. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: an X-ray and NMR investigation. J Am Chem Soc. 2009 Apr 15;131(14):5153-62. PMID:19309137 doi:10.1021/ja808285e
Proteopedia Page Contributors and Editors (what is this?)
Hannah Campbell, Michal Harel, Tihitina Y Aytenfisu, Alexander Berchansky, Joel L. Sussman, Sandra B. Gabelli